order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Location
Corporate Headquarters
Vector Laboratories, Inc.
6737 Mowry Ave
Newark, CA 94560
United States
Telephone Numbers
Customer Service: (800) 227-6666 / (650) 697-3600
Contact Us
Additional Contact Details
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
order histories, retained contact details for faster checkout, review submissions, and special promotions.
7 Steps to Immunohistochemistry Validation
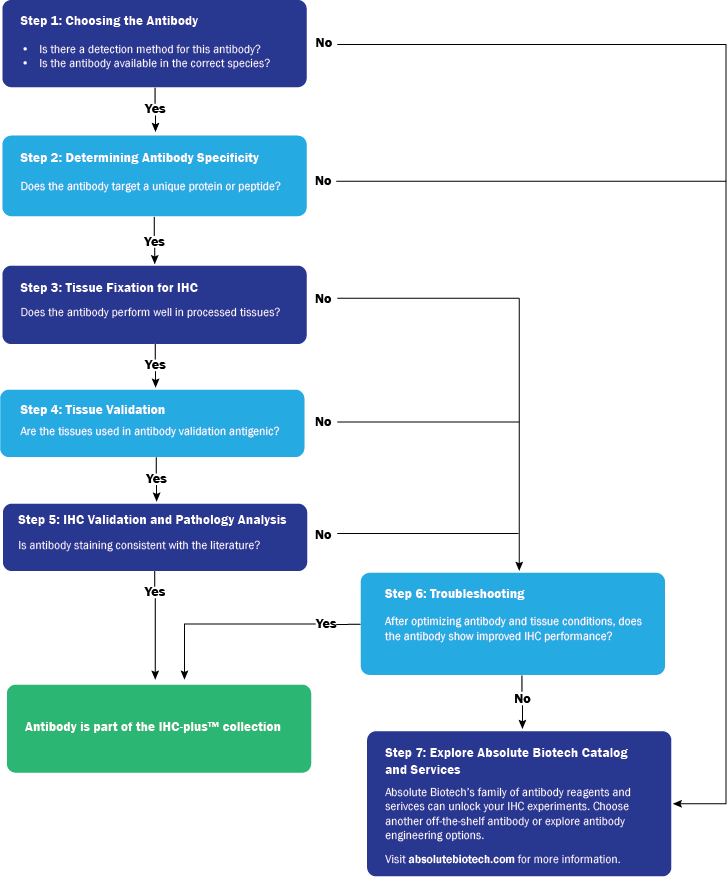
As antibody experts, we are continually amazed by the research and diagnostic opportunities enabled by the specificity of antibodies and antigens. Immunohistochemistry (IHC) is one of the processes that leverage antibodies’ unique binding properties to image and identify what cannot be seen by the naked eye or under a microscope alone. The method uses antibodies to localize or identify the presence of antigens in cells and tissues, from amino acids and proteins to infectious agents and specific cellular populations.
IHC is an important tool for both clinicians and scientific researchers. For the clinician, the IHC method allows for the diagnosis of specific diseases and histological origins of cells that cannot be determined by morphological visualization. In the research community, the strength of immunohistochemistry to localize the expression of a specific target protein is a valuable tool. Tumor biomarkers, for example, are one of the categories of antigens commonly stained for using IHC-validated antibodies.
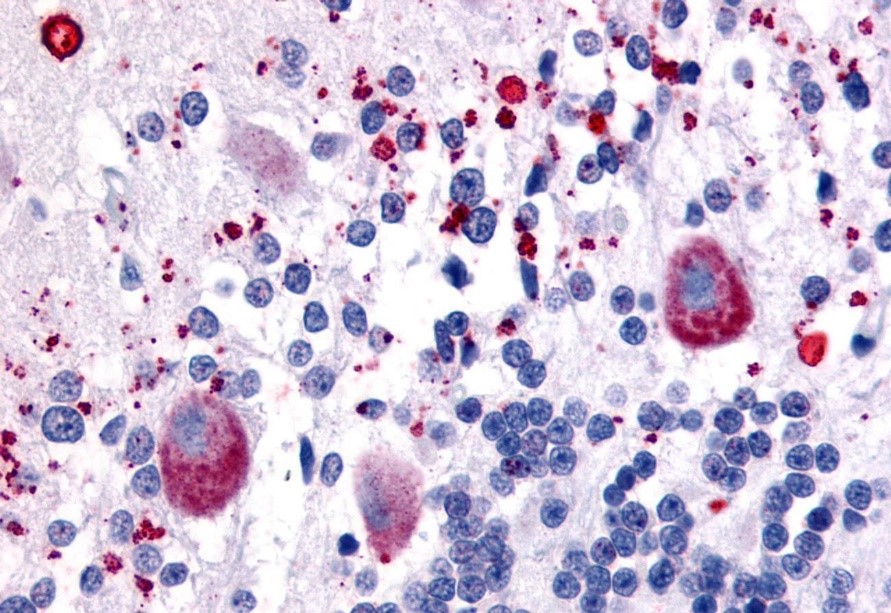
Not all antibodies are equal to the task of immunohistochemical staining, however. Many antibodies that perform well in other assays do not work well in IHC against formalin-fixed paraffin-embedded tissues. In immunohistochemistry, antibodies may produce weak or no signal, show nonspecific background staining that interferes with analysis, or cause false positive signals. Therefore, IHC experiments must be run with the proper positive and negative controls and the results then compared to the literature to determine if the antibody is giving the expected staining pattern. If scant publications are available for a target, then alternate antibodies raised against a different portion of the same target can be run to confirm the localization.
Over the past 20 years, we have designed and conducted thousands of IHC experiments, with the goal of identifying the antibodies that perform the best in IHC against formalin-fixed paraffin-embedded (FFPE) human tissues. Our validation protocol has been used to test monoclonal antibodies (mouse, rabbit, rat), polyclonal antibodies (rabbit, goat, sheep, llama, chicken), and human single- and double-chain antibodies for use in IHC. We offer the highest performing antibodies as part of the IHC-Plus™ product line, to ensure you make the most of your time and tissue samples.
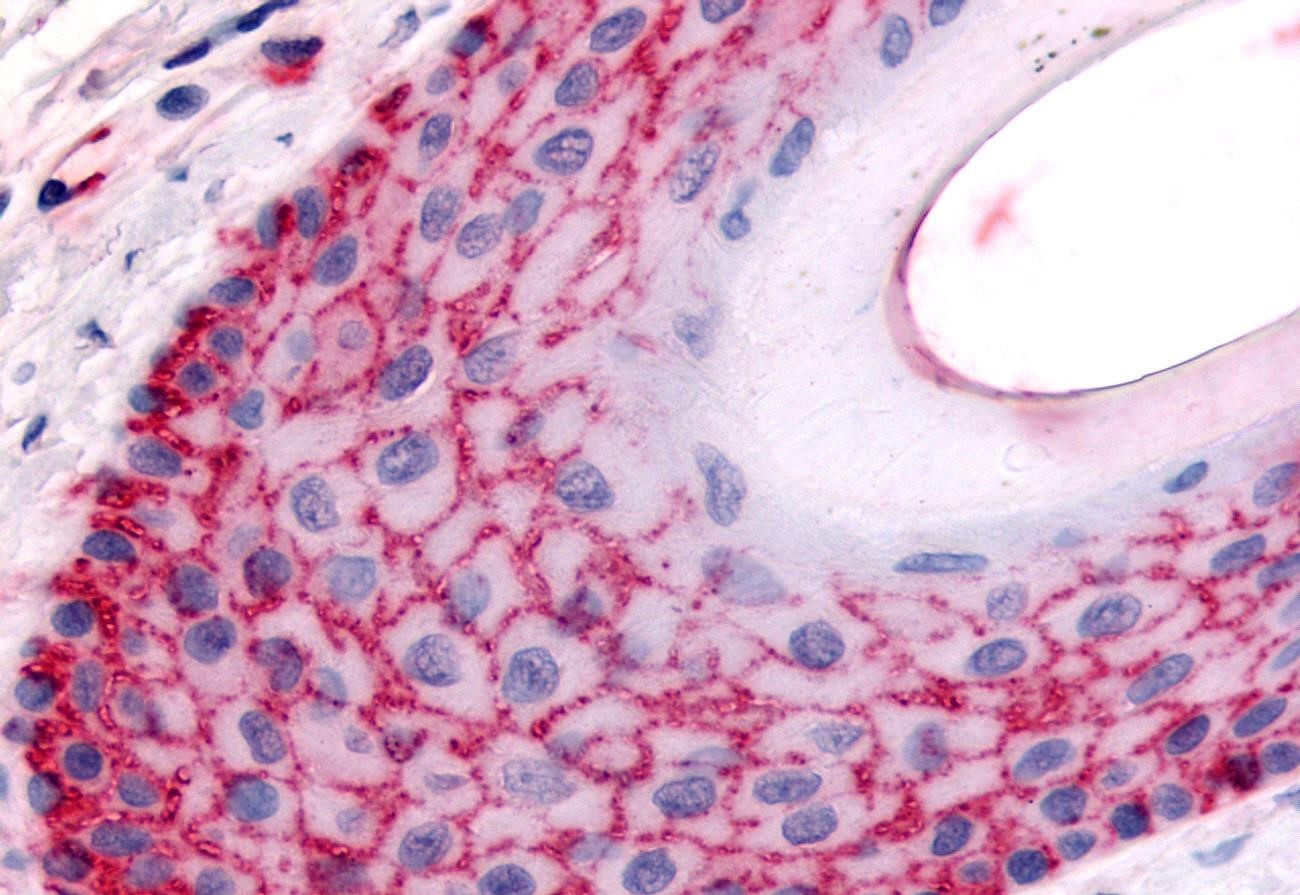
The following is our methods and approach to IHC antibody validation. We use this process for deciding which antibodies join the ranks of our IHC-Plus™ products, antibodies you can trust to perform in IHC assays.
Before the validation process begins, promising antibodies must be considered according to a simple question: can this antibody be used to stain tissues? Namely, is there a detection method for this antibody? Any antibody that has a secondary antibody or reagent that allows detection of the primary antibody is a candidate for use in IHC. The next question to consider is whether this antibody’s detection method is appropriate.
For the most specific staining, it is best to use antibodies that are raised in a different species from the species of the target tissues. This reduces background staining and produces cleaner data. The secondary antibody can be conjugated with a colorimetric detection system such as horseradish peroxidase-DAB or alkaline phosphatase-Vector Red detection systems. For antibodies that target antigens in the presence of natural pigments (e.g., melanin, hematin, carbon), care must be taken to ensure the detection system does not use a similar wavelength or have the same color as these pigments, or the IHC signal may be masked.
However, there are cases where the antibody will be the same species as the target tissue (e.g., humanized mouse antibodies in human tissues). In these cases, our antibody experts have chosen to incorporate a tag, such as FITC, His, or Biotin, and then choose a secondary detection antibody that targets the tag, rather than the species backbone. This usually results in cleaner data. Our IHC-Plus™ antibodies take all these questions into account when we select which antibody clones get validated and added to our catalog, further ensuring that the researchers we supply can focus on their projects, not shopping around for appropriate reagents.
For researchers choosing which antibody reagents to purchase, it is important to consider antibody specificity beyond simply matching antibody to antigen. When validating antibodies for IHC applications, our antibody experts perform tests that ensure that our IHC-Plus™ antibodies are specific and selective, only targeting unique antigens, thereby reducing nonspecific background staining and interference with pathologic interpretation.
If the antibody targets a protein peptide sequence, it is crucial the peptide be unique to the target protein and not appear in any other protein sequences. To accomplish this, we run BLAST sequence similarity searches on peptides in question and determine if there are any unwanted proteins or parts of proteins that may react with this antibody. Only antibodies that are highly selective and specific to the target peptide are moved forward in the antibody validation process.
If the antibody targets a protein (either a short piece or the full length of the protein), we must ensure the antibody is specific in practical assays. One such method is running a Western blot using the antibody of interest. We find, however, that Western blots can be poor indicators of IHC performance; we have seen antibodies that perform badly in Western blot but stain beautifully in IHC applications, and vice versa. The more direct method we use for determining antibody specificity is to use the antibody in IHC or immunocytochemistry (ICC) on over-expressing cell lines or short-term transfected cells, and then compare the results to the same antibody used in negative control cell lines.
Researchers who use IHC are sure to have encountered the trade-offs that preserving and analyzing tissues require; on one hand, antigens are the easiest for antibodies to identify in tissues that are fresh or frozen, while on the other hand, tissue morphology is best preserved with fixatives that cause cross-linking or denaturing. Antigen retrieval methods that undo the cross-linking that fixatives cause are effective at restoring a degree of immunogenicity, but occasionally, antibodies do not work in fixed tissues at all.
Monoclonal antibodies are advantageous for most antibody applications due to their high degree of biological definition and specificity, but they can often underperform or not perform at all in human tissues that have been formalin fixed. About half of all monoclonal antibodies that we test using formalin-fixed tissues show a diminished signal compared to polyclonal antibodies targeting the same antigen. However, when monoclonal antibodies show a strong signal in formalin-fixed tissues, they are the preferable reagent as their high specificity allows for reduced nonspecific background.
Polyclonal antibodies show a higher signaling success rate when used in formalin-fixed tissues; we observe a 60-75% success rate depending on the target. However, they are not our first choice for IHC applications, as the polyclonality may reduce specificity and increase nonspecific background. However, high-affinity polyclonal antibodies can be used at reduced concentration there by decreasing nonspecific binding and IHC data analysis can include nonspecific background mitigation.
Our antibody experts know the value of high-quality controls, reagents, and procedures. To maintain our exceptional validation standards, we ensure that the formalin-fixed tissues we test our antibodies on are antigenic after antigen retrieval and that the signal strength is consistent with what we expect from these tissue sections. Some of the antibodies we use to test our tissues target cytokeratin, vimentin, Factor VIII, CD31, CD3, CD20, or GFAP. If a tissue does not show the expected signal strength typical for the test antibody in that cell type, it is rejected. If a tissue passes muster, then we proceed with IHC-Plus™ validation and apply our antibody candidate to the tissue.
Every antibody that undergoes LSBio’s IHC validation process gets tested at a minimum of four concentrations on a multi-tissue formalin-fixed, paraffin-embedded panel of 22 normal human tissues. In many cases, three brain regions or multi-cancer array are added to supplement testing of relevant antibodies. Antibody performance is then scored for each tissue and cell types for a comprehensive view of the antibody’s behavior, sensitivity, specificity, and selectivity.
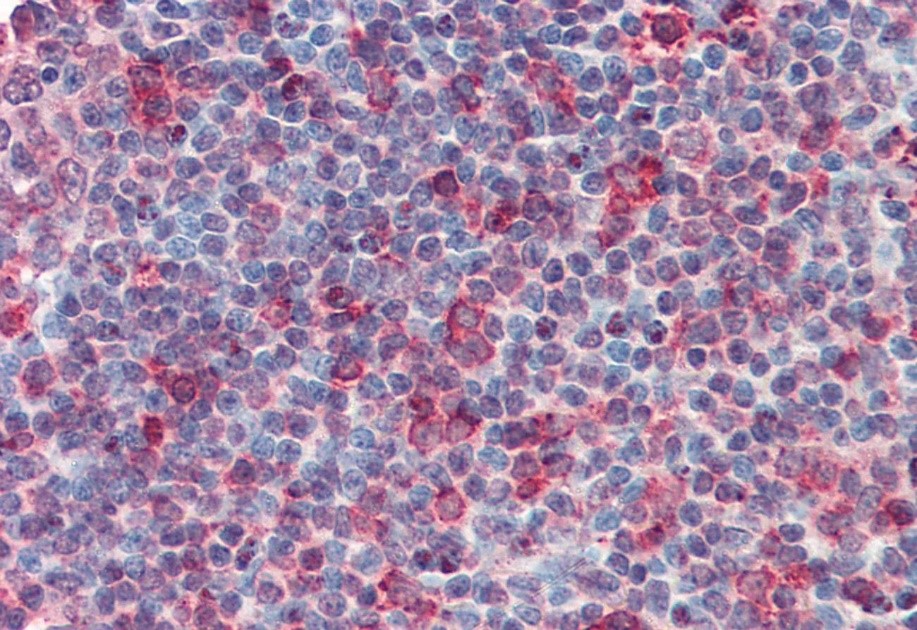
The next step is to ensure that our results align with current literature on the antibody target. If our results align with the expected outcome according to the body of data, we add the antibody to our ranks of IHC-Plus™ antibodies. The data and ratings we compile for each antibody are available on the antibody’s product page. Further information on our protocols and ratings algorithm can also be found on each product’s page. We do the work so you can feel confident employing our antibodies in your IHC experiments.
For the antibodies that fail any part of the validation, we must make sure the results accurately reflect the antibody’s IHC potential. An antibody that is too dilute or low affinity for the target may falsely read as negative. An antibody that has not been affinity purified may produce too much nonspecific background. The antigen retrieval process in the tissues may not be optimal for the specific antibody. If the antibody we are validating is high value and does not have reliable alternatives, we will explore all the troubleshooting options to ensure that our IHC-Plus™ antibodies are high-quality and that they enable the full breadth of research possibilities for our customers.
If you are a researcher who is pushing the currently published boundaries of protocol and experimental design, you may be frustrated at the lack of available antibodies that fit your exact needs. Our IHC-Plus™ collection includes more than 15,000 antibodies validated for immunohistochemistry, but that still does not guarantee that you have exactly what you are looking for.
Luckily, LSBio is part of the Absolute Biotech brand of antibody-focused companies. Contact us for more information on our antibody offerings or take a look at the custom recombinant engineering options from our sister company, Absolute Antibody. Whether in live animal models or fixed tissues, our antibody engineers work with researchers to craft optimal reagents for their projects.
With more than two decades and thousands of experiments devoted to finding the right antibodies against FFPE tissues, we are proud to provide our validated antibodies to researchers. Benefit from standardized validation protocol, well-characterized results, accessible usage information, and clean data with LSBio’s IHC-Plus™ family of antibodies. We take the guesswork out of your choice of IHC reagents, saving your time and tissues. Click here to explore the full breadth of thousands of IHC-validated antibodies against a wide array of targets. If you are not sure where to start or would like to receive a quote, contact us! Our antibody experts are happy to answer any questions you may have.










