order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Location
Corporate Headquarters
Vector Laboratories, Inc.
6737 Mowry Ave
Newark, CA 94560
United States
Telephone Numbers
Customer Service: (800) 227-6666 / (650) 697-3600
Contact Us
Additional Contact Details
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
order histories, retained contact details for faster checkout, review submissions, and special promotions.
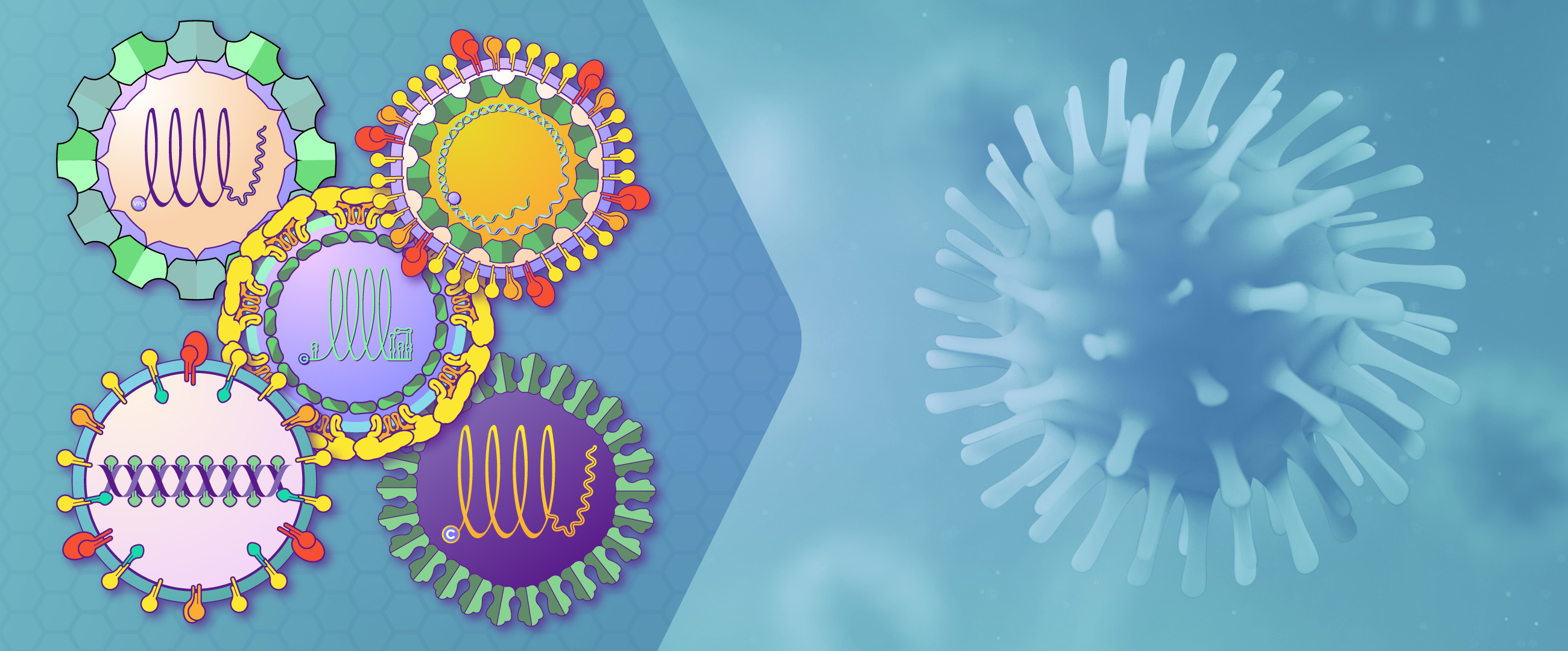
Hepatitis Viruses
Hepatitis viruses cause 1.3 million deaths worldwide annually, and cause liver diseases including acute and chronic infections, liver failure, cirrhosis, and hepatocellular carcinoma. These viruses are a diverse group, and can be either dsDNA viruses such as Hepatitis B virus (family Hepadnaviridae), or ssRNA viruses such as Hepatitis A (family Picornaviridae), Hepatitis C (Flaviviridae), Hepatitis D (Deltavirus) and Hepatitis E (Hepeviridae).
About Hepatitis Viruses
They all have in common their ability to infect hepatocytes and cause jaundice and liver disease (Table 1 from Rasche 2019):
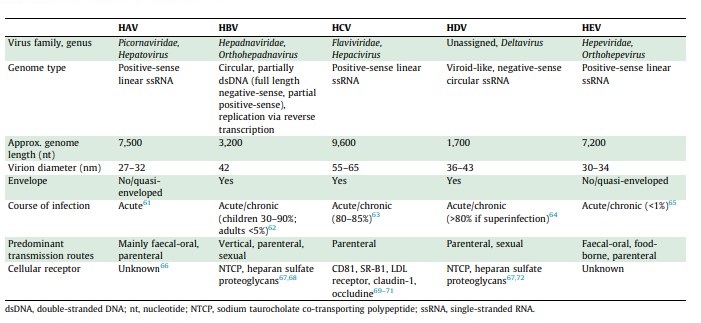
Table 1: Properties of Hepatitis Viruses
Hepatitis A
Hepatitis A virus belongs to the family Picornaviridae, which contains non-enveloped positive-sense ssRNA viruses that also include members that infect respiratory epithelium and cause the common cold (Rhinovirus), and those that infect the CNS cause meningitis and paralysis such as poliomyelitis (Poliovirus). Other diseases caused by Picornaviridae include encephalomyocarditis and foot-and-mouth disease (Gorbalenya 2020). This family includes important human and veterinary pathogens that can infect the nervous system, heart, liver, skin, gastrointestinal and respiratory tracts.
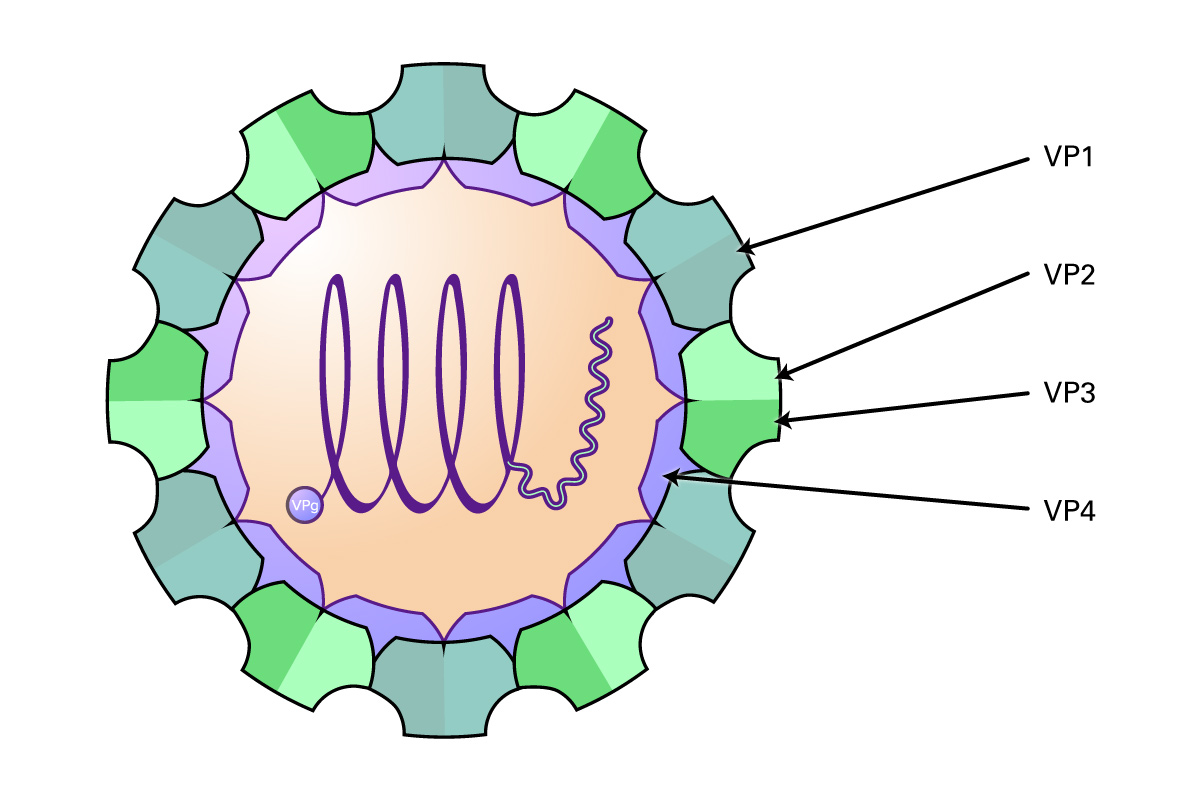
These viruses are approximately 30 nm in diameter, and contain an icosahedral capsid made of four proteins (VP1-4) that surround the 6-10 kb RNA genome. Proteins VP1-3 are located on the external side of the capsid, and VP4 is located internally. The genome consists of a single ORF encoding three groups of proteins: the P1 region which encodes structural polypeptides (L, VP1-3), and the P2 (2A, 2B and 2C) and P3 regions (3A, VPg, 3Cpro, RdRp) encoding proteins required for viral replication. In addition, the virus contains a long UTR at the 5’ end containing an internal ribosome entry site that allows direct translation of the polyprotein, and a shorter 3’ UTR that is important in negative-strand RNA synthesis. Some genera also contain an N-terminal leader protein (L) that can be a papain-like cysteine proteinase or perform some other function. After the virus attaches to host receptors and enters via endocytosis, the VP4 protein opens a pore in the host endosomal membrane, allowing the genomic RNA to enter the host cytoplasm. VPg is removed from the viral RNA, which is translated into the polyprotein. Replication occurs in membrane vesicles derived from the ER where dsRNA is initially synthesized, then transcribed and replicated into ssRNA(+) genomes and packaged into preassembled procapsids. Virus is then released via cell lysis.
Hepatitis A virus differs from other picornaviruses in that it is non-cytopathogenic and particles are released within membranous structures containing 1-4 virions. The virus infects the epithelial cells of the small intestine and hepatocytes of primates. Viral replication predominantly occurs in the liver, is excreted via the bile into the colon, and transmitted fecal-orally. Clinical manifestations include jaundice, fever, abdominal pain, light stools, and diarrhea. The infection is generally of a limited nature, and does not persist chronically. The virus is very stable, resistant to acid pH, and stable at elevated temperatures (60 degrees C) for short durations.
Hepatitis B
Hepatitis B virus (HBV) is a member of the Hepadnaviridae family of partially double stranded, enveloped DNA viruses with a circular genome and an icosahedral capsid. The family includes include Orthohepadnaviruses and Avihepadnaviruses. These viruses infect hepatocytes and cause liver injury and hepatocellular carcinomas in mammals and birds.
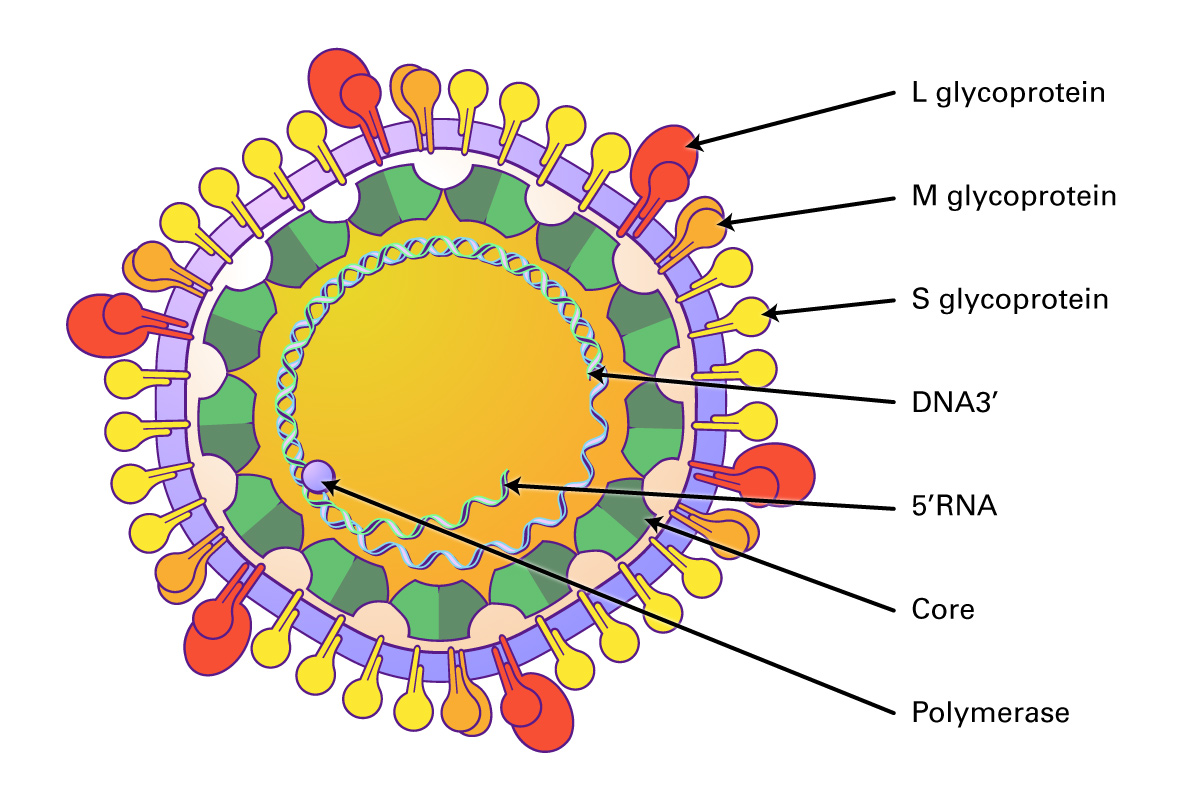
These viruses are approximately 30 nm in diameter, and contain an icosahedral capsid made of four proteins (VP1-4) that surround the 6-10 kb RNA genome. Proteins VP1-3 are located on the external side of the capsid, and VP4 is located internally. The genome consists of a single ORF encoding three groups of proteins: the P1 region which encodes structural polypeptides (L, VP1-3), and the P2 (2A, 2B and 2C) and P3 regions (3A, VPg, 3Cpro, RdRp) encoding proteins required for viral replication. In addition, the virus contains a long UTR at the 5’ end containing an internal ribosome entry site that allows direct translation of the polyprotein, and a shorter 3’ UTR that is important in negative-strand RNA synthesis. Some genera also contain an N-terminal leader protein (L) that can be a papain-like cysteine proteinase or perform some other function. After the virus attaches to host receptors and enters via endocytosis, the VP4 protein opens a pore in the host endosomal membrane, allowing the genomic RNA to enter the host cytoplasm. VPg is removed from the viral RNA, which is translated into the polyprotein. Replication occurs in membrane vesicles derived from the ER where dsRNA is initially synthesized, then transcribed and replicated into ssRNA(+) genomes and packaged into preassembled procapsids. Virus is then released via cell lysis.
In humans, HBV infection is typically transmitted via blood transfusion. The HBV vaccine can prevent disease, and nucleoside analogs (lamivudine, entecavir, adefovir, telbivudine, tenofovir) have been used for long term antiviral therapy to suppress viral genome replication (Yuen 2018).
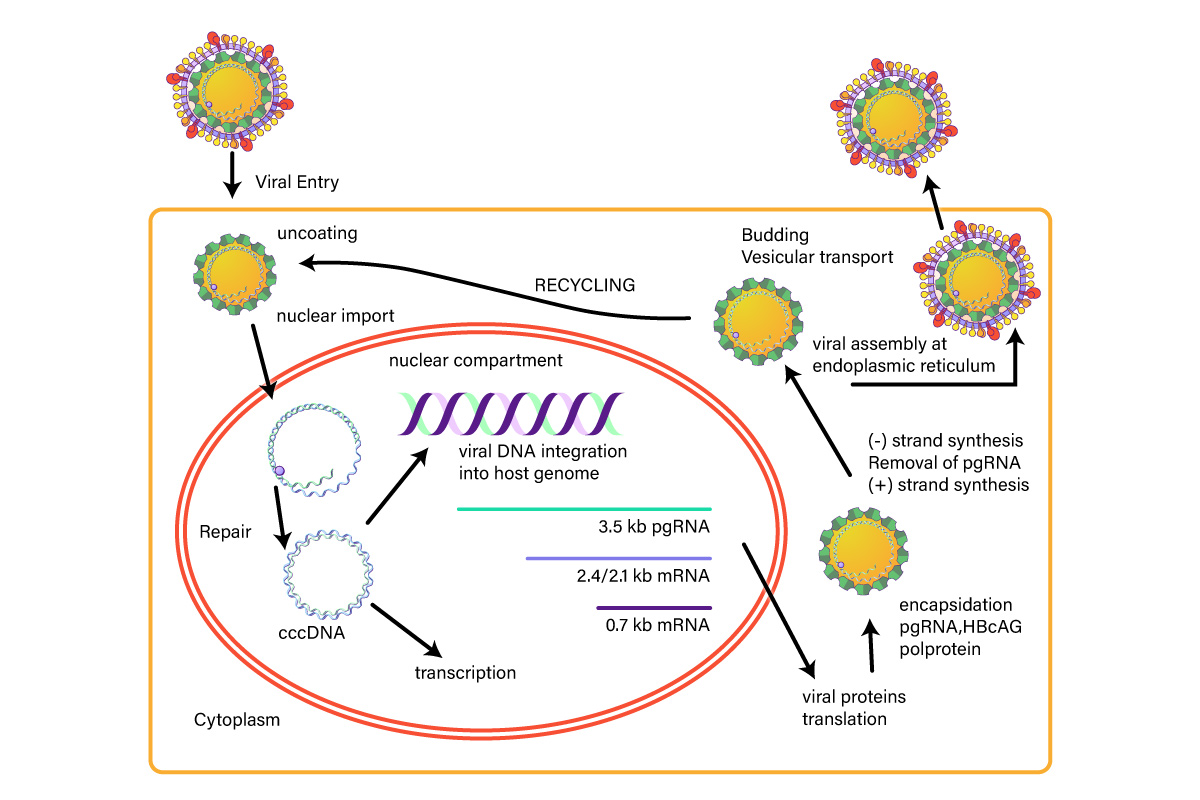
Figure 1: Hepatitis B Life Cycle
Hepatitis C
Hepatitis C virus is a small, enveloped, icosahedral, positive ssRNA virus that belongs to the Flaviviridae family. The Flaviviridae are vector-borne RNA viruses that cause a wide spectrum of diseases including hepatitis, encephalitis, vascular shock, acute flaccid paralysis, congenital abnormalities, and fetal death (Pierson, 2020). The family is subdivided into three genera: Flavivirus, Pestivirus, and Hepacivirus. The Flavivirus genus are zoonotic and insect-borne viruses, and responsible for Yellow fever, Dengue fever, Japanese encephalitis, West Nile encephalitis, and tick-borne encephalitis. The Pestivirus genus includes animal viruses causing bovine viral diarrhea and classical swine fever. The Hepacivirus genus (Hepatitis C) causes viral hepatitis and hepatocellular carcinoma in humans.
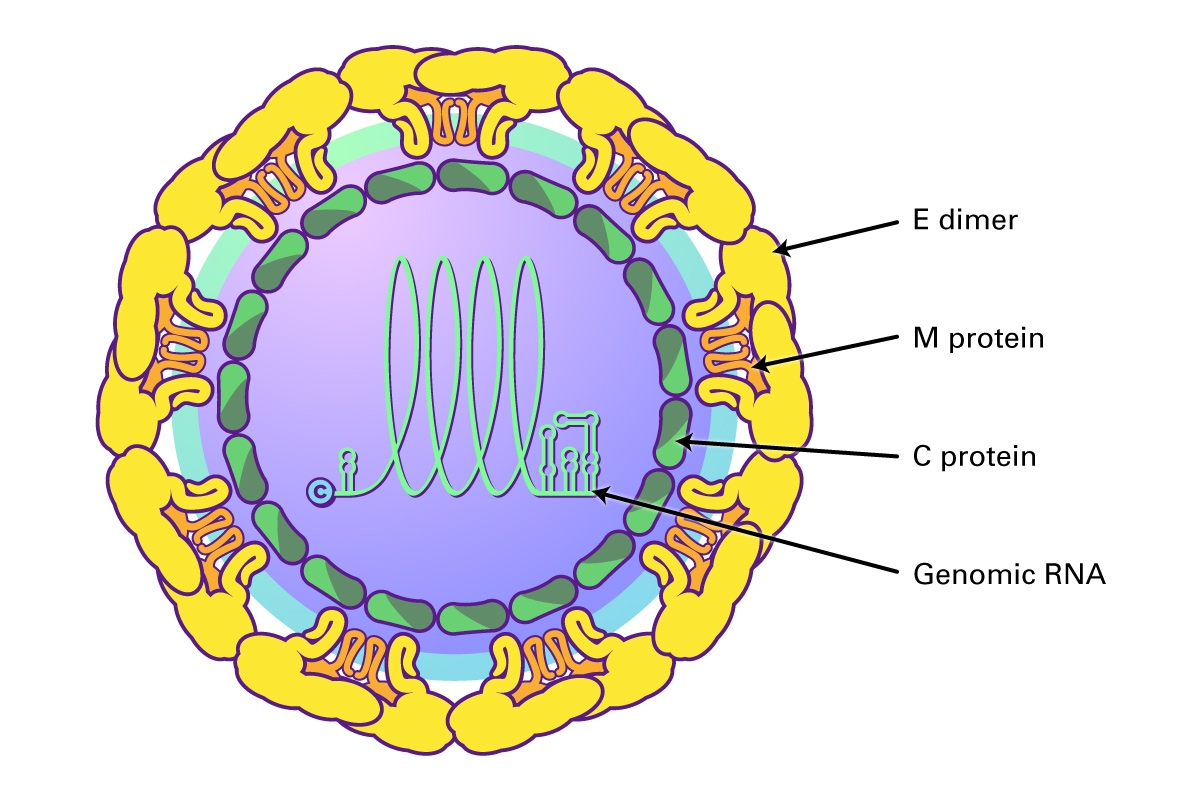
Flaviviruses share a common organization; they encode a single open reading frame that is translated into the ER as a polyprotein, which is cleaved by viral and host proteases into ten functional proteins, including three structural proteins (C, prM and E) and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A and B, and NS5 polymerase). The virus is assembled using the three structural proteins, a host envelope, and viral genomic RNA. Non-structural (NS) proteins include those involved in viral replication, such as the RNA-dependent RNA polymerase.
Hepatitis C virus (genus Hepacivirus) binds to two cell surface receptors, the low density lipoprotein receptor (LDLr) and heparin sulfate proteoglycans (HSPGs). This triggers the HCV outer E1/E2 heterodimer to bind to the scavenger receptor B1 and tetraspanin protein CD81. CD81 then interacts with claudin-1, triggering clathrin-mediated endocytosis and endosomal fusion. The released viral RNA is then translated and replicated via the NS5B RNA-dependent RNA polymerase to produce a negative-sense RNA template from which multiple positive-sense progeny are replicated. The newly synthesized HCV RNAS are then incorporated into nucleocapsid particles, fuse with a luminal lipid droplet containing ApoE proteins to create a high density HCV precursor, and then transit along with the VLDLs formed in the Golgi as multi-vesicular bodies to the cell surface where they are released as a lipoviral particle after fusing with the hepatocyte cell membrane (Dubuisson, J 2014; Grassi 2016).
Hepatitis C infection is the leading cause of liver-related mortality, and chronic infection can lead to cirrhosis, liver failure, hepatocellular carcinoma, liver transplants, and death. Direct-acting antivirals (DAAs), drugs that target multiple mechanisms of the HCV life cycle, have been successful in eradicating HCV from most patients, including those co-infected with HIV (Marks 2019, Morozov 2018). These include sofosbuvir/velpatasvir, which combines and NS5A and NS5B inhibitors, or glecaprevir/pibrentasvir, both of which are pangenotypic treatments that can be dosed once daily for twelve weeks for viral eradication.
Hepatitis D
Hepatitis delta virus (HDV) is a defective RNA virus that depends upon the expression of the hepatitis B virus surface antigen (HBsAg) in the same cell in order to complete its life cycle. HDV can infect hepatocytes that do not contain HBsAg, and can replicate its genome and express the hepatitis delta antigen, however, is unable to secreted infectious viral particles. When co-infection occurs with HBV, chronic hepatitis D is the most aggressive form of viral hepatitis, and is associated with cirrhosis, liver failure, and hepatocellular carcinoma. Co-infection can occur simultaneously with HBV, or can occur as a superinfection of a chronic carrier of HBV. Co-infection causes acute hepatitis, but most cases resolve both infections, although the chance of liver failure is higher than with HBV infection alone. In the case of superinfection, most progress to chronic dual infection with both viruses. There are no significant therapies that are successful in treating HDV infection, and nucleoside analogues that are useful for treating HBV have no effect on HDV replication.
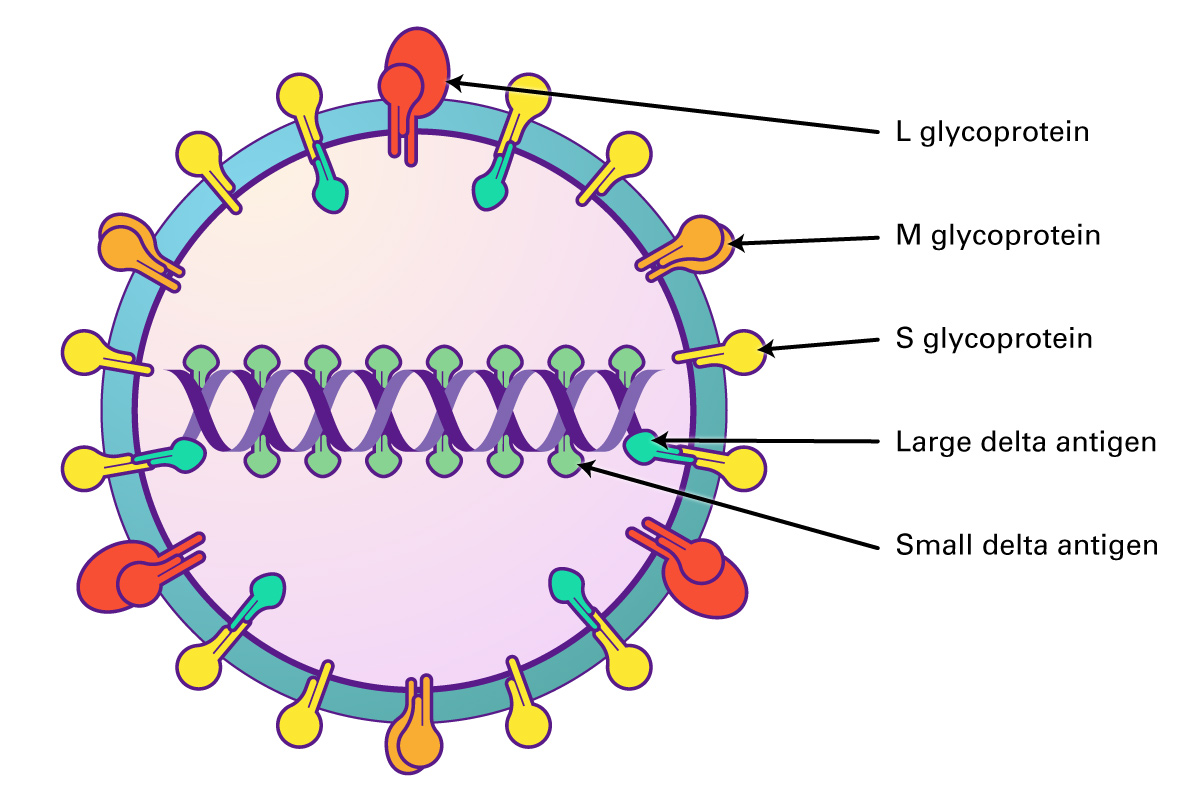
The virions are 35-37 nm, and comprised of an envelope and ribonucleoprotein. Similar in structure to HBV, both viruses use HBV surface proteins S, M, and HBsAg for assembly. The capsid is formed from multimerisation of core protein HBcAG, and contains one copy of the viral circular, ssRNA genome, enclosed by large and small delta antigen which form the viral ribonucleoprotein. The HDV genome is a circular, closed ssRNA which folds into a partially double stranded rod.
The viral life cycle begins when the virus enters hepatocytes through attachment to cell surface heparin sulfate proteoglycans (HSPGs) and SLC10A1, a receptor located at the basolateral membrane and involved in the uptake of bile acids. After entry, the HDV is released into to cytoplasm and transported into the nucleus, where transcription and replication occur. HDV replication is dependent upon host RNA-dependent RNA polymerase and perhaps RNA polymerase II. Replication is via a rolling-circle mechanism, resulting in the production of linear HDV antigenomic RNA, which circularizes and serves a template for genomic-strand RNA. An open reading frame in the antigenome is used to synthesize HDags (S and L-HDAg) that are required for replication and virion assembly. Both proteins then undergo post-translational modifications required for their function. After assembly into RNPs and encapsidation, infectious virions are then secreted via the Golgi or multivesicular body pathway (Mentha, 2019).
Hepatitis E
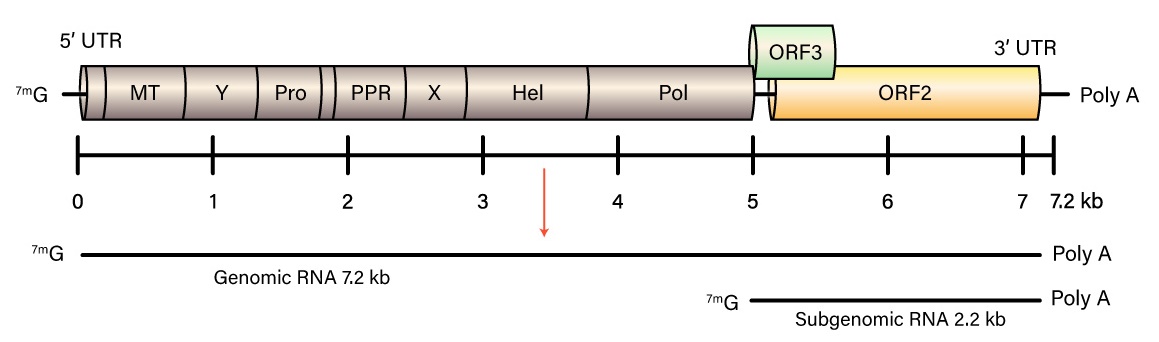
Hepatitis E virus (HEV) is the most common cause of hepatitis globally. A member of the Orthohepevirus family with four species (A to D), human disease is caused by strains of species A. HEV is a non-enveloped virus with an icosahedral capsid, a size of 27 to 34 nm, and contain a positive-sense, single-stranded, 7.2-kb RNA genome which is 5’ capped and 3’ polyadenylated.
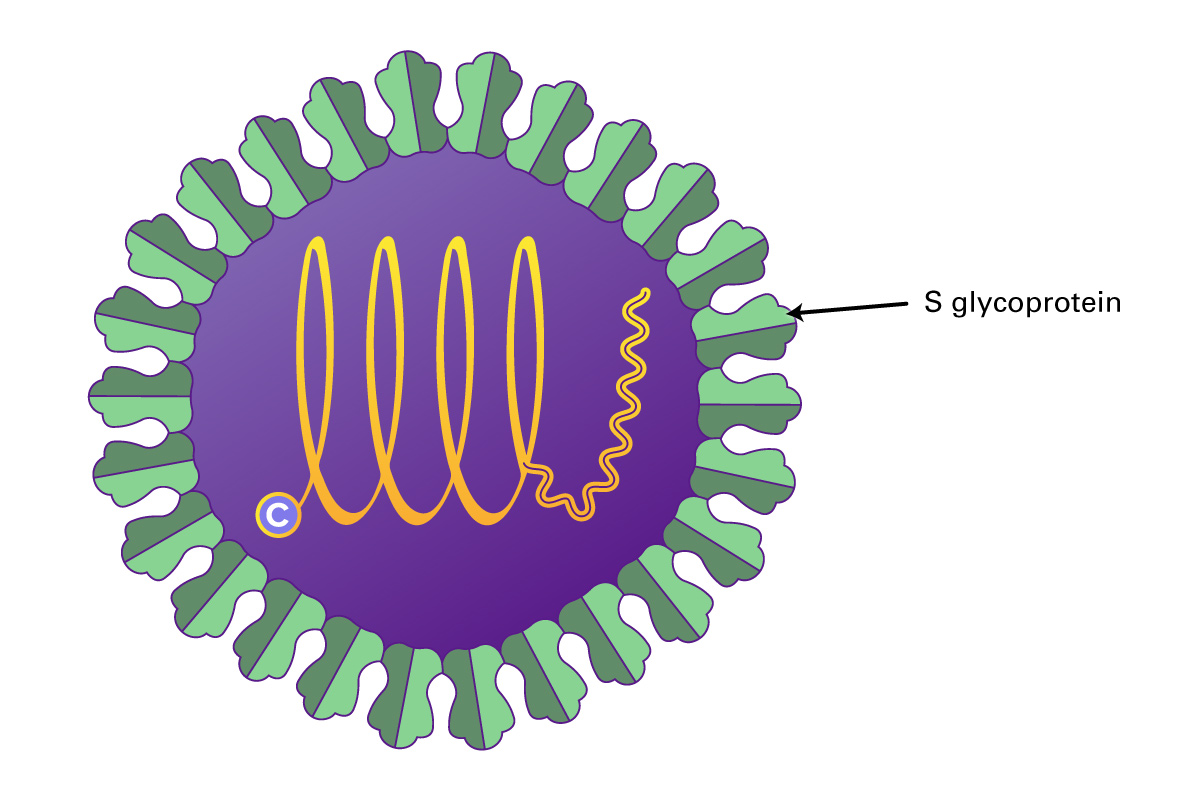
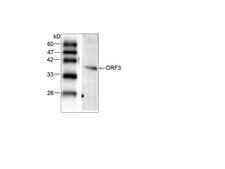
The HEV genome contains three open reading frames (ORFs 1-3). ORF1 encodes a protein of 1,693 amino acids containing functional domains including a methyltransferase, cysteine protease, RNA helicase, and RNA-dependent RNA polymerase. ORF2 encodes the viral capsid protein of 660 amino acids that is responsible for virion assembly, interaction with target cells, and immunogenicity. The ORF2 protein consists of three linear domains: the shell domain (S), the middle domain (M), and the protruding domain (P, harboring the neutralizing epitope(s). ORF3, which overlaps ORF2, encodes a small protein of 113 or 114 amino acids that is involved in virion morphogenesis and release. HEV replicates in the cytoplasm, with a subgenomic RNA producing the ORF2 and ORF3 proteins and the full genomic RNA encoding nonstructural proteins and serving as a template for replication. HEV replicates in hepatocytes but also in the small intestine, colon, and lymph nodes. It can transmit disease either via the fecal-oral route, or through animal reservoirs.
HEV infection causes an acute, self-limited hepatitis in 5% of patients who contract the virus, but can have high mortality in pregnant women, the elderly, or those with pre-existing liver disease. Chronic infection can occur in the immunosuppressed or organ transplant patients. The virus can also cause extrahepatic manifestations including glomerulonephritis, pancreatitis, thrombocytopenia, autoimmune condition, and neurological conditions such as neuritis, neuropathy, Guillain-Barre syndrome, and encephalitis/myelitis.
References
- Rasche, A et al. Evolutionary biology of human hepatitis viruses. (2019) J. of Hepatology. 70(3):501-520. doi.org/10.1016/j.jhep.2018.11.010
- Cornberg M et al. Real-world safety, effectiveness, and patient-reported outcomes in patients with chronic hepatitis C virus infection treated with glecaprevir/pibrentasvir: Data from the German Hepatitis C-Registry. (2019) The International Liver Congress, Vienna, abstract GS-07.
- Dubuisson, J, and Cosset, FL. Virology and cell biology of the hepatitis C virus life cycle: an update. (2014) J. Hepatol 61:S3-S13. doi.org/10.1016/j.jhep.2014.06.031
- Kamar, N et al. Hepatitis E Virus Infection (2014) Clin Microbio Reviews 27(1):116-138. doi.org/10.1128/CMR.00057-13
- Grassi, G et al. Hepatitis C virus relies on lipoproteins for its life cycle. (2016) World J Gastoenterol 22(6):1953-1965. https://doi.org/10.3748/wjg.v22.i6.1953
- Mangia A et al. Global real-world evidence of sofosbuvir/velpatasvir as a simple-effective regimen for the treatment of chronic hepatitis C: integrated analysis of 12 clinical practice cohorts. (2019) The International Liver Congress, Vienna, abstract GS-01.
- Marks, K and Naggie, S. Management of hepatitis C in 2019. (2019) JAMA 322(4):355-356. https://doi.org/10.1001/jama.2019.5353
- Mentha, N et al. A review on hepatitis D: From virology to new therapies. (2019) J. Advanced Research 17:3-15. https://doi.org/10.1016/j.jare.2019.03.009
- Morozov, VA and Lagaye, S. Hepatitis C virus: Morphogenesis, infection, and therapy. (2018) World J. Hepatol; 19(2):186-212. https://doi.org/10.4254/wjh.v10.i2.186
- Pierson, TC and Diamond, MS. The continued threat of emerging flaviviruses. (2020) Nature Microbiology Vol 5, June: 796-812. https://doi.org/10.1038/s41564-020-0714-0
- Webb, GW and Dalton, HR. Hepatitis E: an underestimated emerging threat (2019) Ther Adv Infectious Dis 6:1-18. https://doi.org/10.1177/2049936119837162
- Yuen, MF et al. Hepatitis B virus infection. (2018). Nat Rev Dis Primers 4(4):18035. https://doi.org/10.1038/nrdp.2018.35
- Zell r. et al. ICTV Virus Taxonomy Profile: Picornaviridae. (2017) J. Gen Virology 98:2421-2422. https://doi.org/10.1099/jgv.0.000911
All Hepatitis Viruses