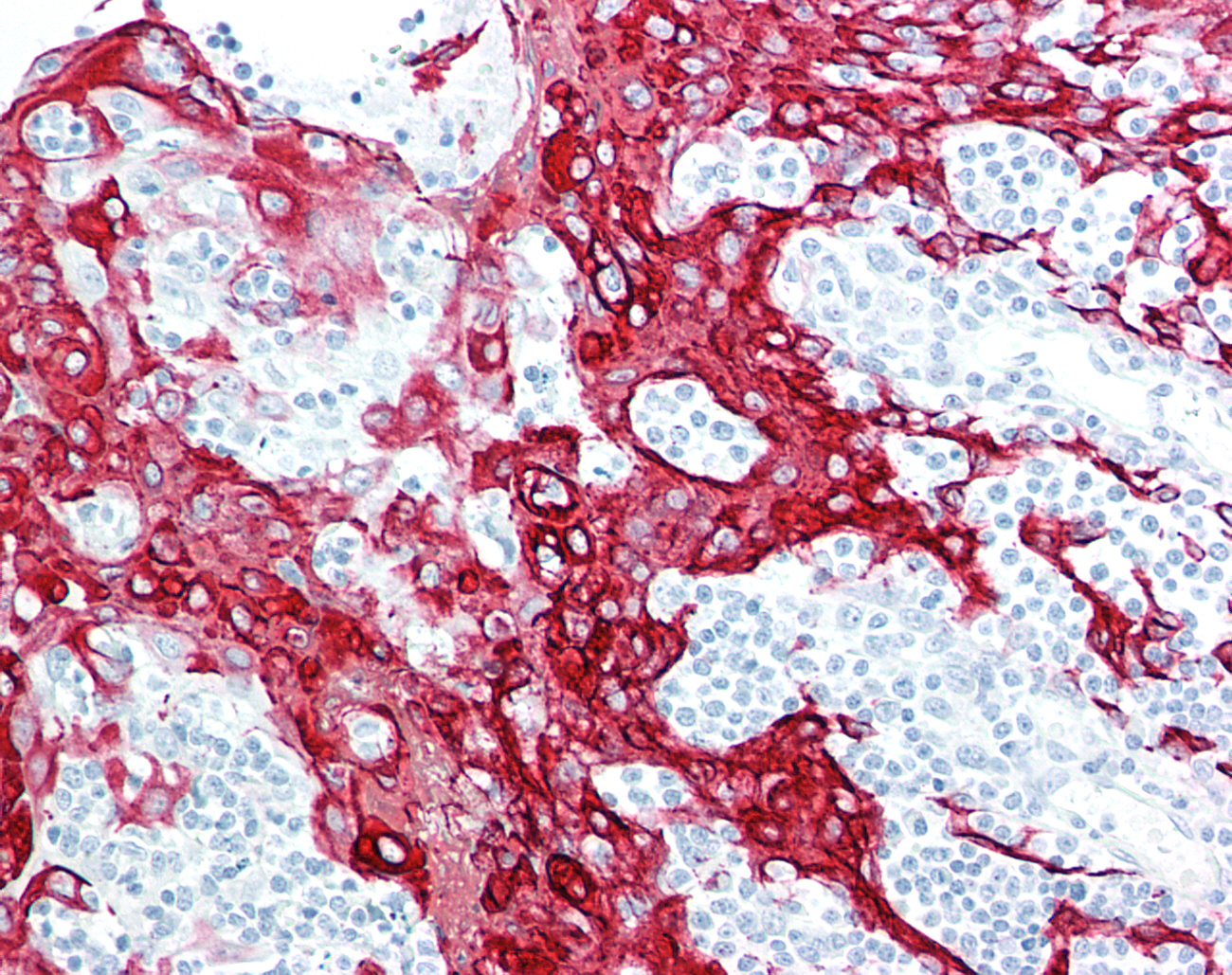
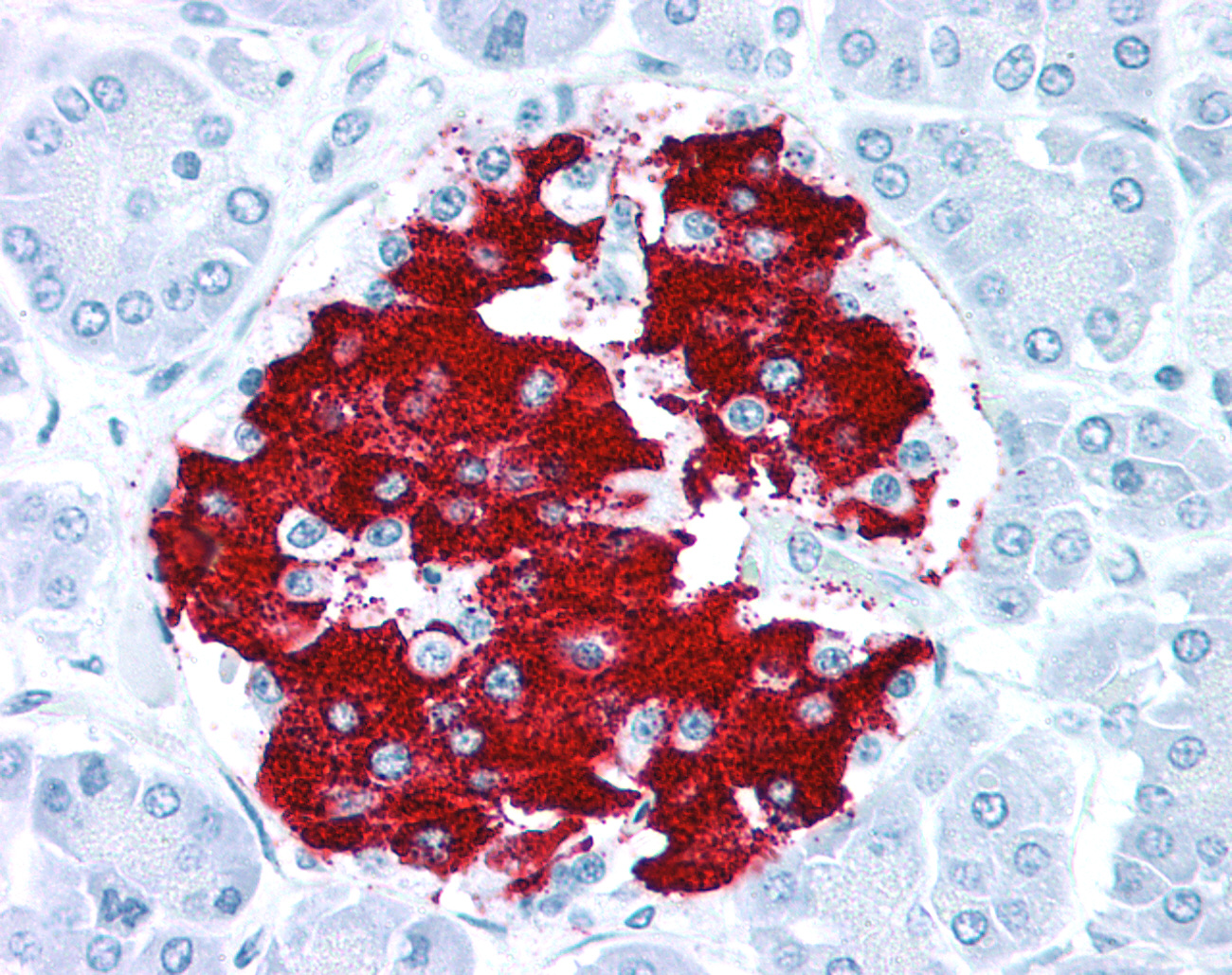
Immunohistochemistry can be performed with any type of antibody for which there is a secondary antibody or reagent to detect the presence of the primary antibody, through either its backbone or via a tag, on cells or tissues.To reduce background staining it is generally preferable to use an antibody generated in a species other than that of the target tissue.For example, to detect a target in human tissues with a rabbit polyclonal or mouse monoclonal antibody, a secondary antibody and detection system may include using an anti-rabbit secondary or anti - mouse monoclonal secondary, followed by using either a horseradish peroxidase-DAB or alkaline phosphatase - Vector Red detection system to produce the colorimetric signal. Although HRP-DAB is as sensitive as AP - Vector Red as a colorimetric detection system, the presence of melanin, hematin, carbon, lipofuscin, and other natural brown or yellow colored pigments within tissues can mask the IHC signal, so for most of our IHC validation work, we use AP - Vector Red.
In cases where the primary antibody backbone species is the same as the target tissue (for example, using humanized antibodies on human tissue), we prefer to have these antibodies conjugated with a tag (FITC, Biotin, Myc, His), so that an anti-tag antibody can be used for detecting the target. This avoids the problem of detecting endogenous immunoglobulins that are detected by the secondary antibody. Although blocking reagents are used during the immunohistochemistry staining procedure, these reagents can often reduce both background as well as signal, so in our experience, the sensitivity is often better preserved by using a tag rather than targeting the primary antibody Fc fragment of the same species.
When purchasing antibodies or synthesized custom antibodies to a target, the most important factor that determines antibody specificity is the immunogen that was used to generate the antibody. When the antibody targets a specific peptide, the peptide should be unique to the target protein and should not contain regions that are either homologous to other members of its protein family or to other proteins that are highly expressed within a cell. LSBio routinely BLASTs peptide sequences to identify proteins that may cross react with the antibody, and excludes peptide sequences or antibodies that may bind regions that contain those sequences. Even if a peptide appears unique, because the epitope-binding site within tissues is often conformational, antibodies can bind nonspecifically to regions of other proteins that mimic the structure of the peptide antigen. These binding events produce "nonspecific" or "background" staining within tissues that may interfere with pathologic interpretation.
In other cases, and frequently with monoclonal reagents, antibodies have been generated to short stretches of a protein or a full-length protein, in which case specificity must be determined by an independent method, such as a Western blot or testing the antibody in immunohistochemistry on over-expressing or short-term transfected cell lines compared to control cells that lack the gene. It is important to note that these methods provide evidence that the target protein is being detected (sensitivity) but do not prove that another target is not also being detected by the antibody (specificity). This is because Western blots performed by many antibody manufacturers are limited to a small number of over-expressing cell lines, or to one or two tissues that overexpress the protein. Although the gold standard is that the antibody will detect a single band on a Western blot, in LSBio's experience, this occurs most commonly with high copy proteins. We and others have seen many examples of antibodies that perform beautifully and specifically in IHC yet produce weak bands on a tissue Western. Conversely, an antibody that produces a beautiful Western blot band often performs poorly in IHC. Western blots are therefore most useful when they are positive, but less useful as predictors of an antibody's IHC performance when they show weak or multiple bands on cell or tissue extracts.
A more direct test for antibody sensitivity and specificity is to perform IHC or immunocytochemistry (ICC) on over-expressing and negative control cell lines. Short-term transfected cell lines are more frequently positive than stable cell lines in these types of experiments because stable cell lines may express levels of protein that are below the limit of sensitivity of detection for the antibody in IHC. Performing IHC on formalin-fixed, short-term transfected cell lines is also highly predictive of whether or not the reagent will work on formalin-fixed tissues, because the conditions that are used to produce the signal and the fixation condition for the cell lines and the tissues can be experimentally adjusted to parallel one another. These experiments do not address the issue of whether or not the antibody will detect an anomalous protein once it is used on tissues, but they do demonstrate whether or not the antibody is at least detecting the target within cells that are known to express them.
Another assay frequently requested by our customers is the use of peptide or protein to competitively bind the antibody signal in IHC in order to determine if the signal that is detected on a particular cell type can be blocked by the peptide. In our experience, a competitive blocking experiment with a peptide and its antibody can fail to produce useful data on occasion, because many peptides bind nonspecifically to formalin-fixed tissues and can obscure or actually enhance the antibody signal. In LSBio's experience, an IHC blocking experiment is therefore of value only about half the time.
Antigens are preserved in their most natural state in fresh or frozen tissues. Tissue morphology, however, is best preserved by fixing samples in cross-linking or denaturing preservatives such as formalin, paraformaldehyde, alcohol, methyl Carnoy's, picric acid or mercuric-based reagents. After fixation, antigens are often denatured and folded into shapes that are not recognizable by an antibody that has been generated to a native protein. Although steam or microwave-based antigen retrieval methods or the use of Proteinase-K can recover antigenicity in fixed tissues, many antibodies (particularly monoclonal reagents) may not work at all in fixed tissues, even after antigen retrieval.
In our experience, monoclonal antibodies are most frequently positive in tissues that are fresh, frozen, or fixed in acetone for brief periods. With formalin fixation, even after antigen retrieval, we have observed that the majority of commercial mouse monoclonal antibodies will show no signal or a very weak signal in human tissues. Proteinase K can rescue some signals for some antibodies, but about half of these antibodies will still continue to produce a diminished signal compared to frozen tissues or a comparable polyclonal antibody. When monoclonal antibodies do perform well in formalin -fixed tissues, they produce a very low nonspecific background and are often excellent reagents.
Polyclonal antibodies are more frequently positive on fixed tissues (success ranges from 60-75%, depending upon the target class). The drawback of polyclonal antibodies is that they generally produce a higher nonspecific background staining on tissues than monoclonal antibodies, because they detect multiple parts of the immunogen. Frequently observed background reactions include faint nonspecific staining of renal tubules, islets of Langerhans, connective tissue, serum, or smooth muscle. If a polyclonal antibody has a high affinity for its target protein, or the protein is present at a high copy level, the antibody concentration can be reduced to remove or diminish the background staining reaction. Pathologists who have had substantial experience with polyclonal antibodies will often subtract this signal during IHC analysis.
Tissue preserved using fixatives such as paraformaldehyde, methyl Carnoy's, alcohol-based fixatives, or reagents that are advertised specifically for the preservation of mRNA often perform poorly in IHC compared to tissues snap-frozen without any fixation or fixed in neutral buffered formalin.
Prior to testing the antibody, LSBio validates the tissues for preservation of antigenicity after antigen retrieval. Sections from formalin-fixed tissue blocks (multi-tissue or individual tissue) are tested in advance with positive control antibodies that are known to produce a particular strength signal in those tissues. Antibodies that we frequently use for tissue validation include Cytokeratins, Vimentin, CD31 (endothelial marker), CD3 (T Cell marker), CD20 (B cell marker), or GFAP (glial marker for CNS). Tissues are rejected if they do not show the strength of signal that is typical for markers that should be strongly positive in that cell type. The use of positive control antibodies is highly predictive of whether or not a particular tissue will react to a test antibody, and such antibodies are used to validate all tissues blocks that are selected for immunohistochemistry. The same antibodies can also be used for frozen tissues or tissues fixed by any method to determine if the tissue section is showing reactivity with positive control antibodies.
LSBio tests each antibody at a minimum of four dilutions or concentrations on a multi-tissue formalin-fixed, paraffin-embedded tissue array of 22 normal human tissues, and a separate section of 3 brain regions or a multi-cancer array is often included. This fairly comprehensive panel allows the pathologist to create a table of all of the cell types that are positive, the degree to which they are positive on a 0 to 4 scale (0=negative, 1=blush, 2=faint, 3=moderate, 4=strong), a list of tissues and cell types that are negative for staining, and the subcellular or extracellular structure that is showing staining (i.e. cytoplasmic, membranous, nuclear, extracellular, serum, collagen). This analysis provides a comprehensive view of the behavior of the antibody, its sensitivity, its degree of specificity for cell types that are known to be positive, and the level of nonspecific background that the reagent is showing. These results are then compared to known information about the protein within the public domain, or compared to other antibodies to the same target protein. The results allow the pathologist to determine if the antibody is showing staining that is consistent with what is expected for the target. In many cases, the public information on a target is either incomplete, or highly selective (reports on one or two antibodies), but the overall staining pattern for a reagent should be consistent with the literature although any individual antibody is highly likely to show some differences when compared to another antibody to the same target, particularly if the epitope was from a different region of the protein used as the antigen.
Antibodies can fail to perform in IHC for a variety of reasons. If the antibody is too dilute or has a low affinity for its protein, the signal may be falsely negative. If the antibody has not been affinity purified, it may produce a high background on tissues, rendering interpretation difficult. The antibody may detect other proteins besides the target, and produce an anomalous binding pattern in tissues. The antibody may be one of a subset that works best in either frozen or Proteinase-K antigen retrieval rather than steam-based antigen retrieval. Depending upon the initial IHC results, troubleshooting an antibody that is working poorly in IHC is a time-consuming effort, and if other reagents are available to the same target, it may be less expensive and less time-consuming to simply purchase a different product. When we perform contract immunohistochemistry services for our customers, we routinely purchase multiple antibodies to the same target and test them side by side in order to compare the reagents and identify the best performer for a particular study because we have found that it is more cost-effective for our customers.
If an antibody is a particularly valuable reagent, it is possible to improve its signal in IHC by a variety of methods, including changing the antigen retrieval conditions, the pH of the buffer, the exposure time to the primary antibody, or using various blocking methods, but the fundamental distribution of positive staining in tissues is rarely dramatically changed by these experimental procedures. The factors that most dramatically alter an antibody's binding characteristics in IHC are antibody concentration, tissue fixation, and, for fixed tissues, the use of antigen retrieval. If these variables have been tested with the appropriate positive and negative controls, and the antibody continues either to fail to show a signal or shows a high background signal, it is unlikely that manipulating further experimental conditions will improve the signal by very much. At that point, it is time to get another antibody reagent.
Immunohistochemistry can be performed with any type of antibody for which there is a secondary antibody or reagent to detect the presence of the primary antibody, through either its backbone or via a tag, on cells or tissues.To reduce background staining it is generally preferable to use an antibody generated in a species other than that of the target tissue.For example, to detect a target in human tissues with a rabbit polyclonal or mouse monoclonal antibody, a secondary antibody and detection system may include using an anti-rabbit secondary or anti - mouse monoclonal secondary, followed by using either a horseradish peroxidase-DAB or alkaline phosphatase - Vector Red detection system to produce the colorimetric signal. Although HRP-DAB is as sensitive as AP - Vector Red as a colorimetric detection system, the presence of melanin, hematin, carbon, lipofuscin, and other natural brown or yellow colored pigments within tissues can mask the IHC signal, so for most of our IHC validation work, we use AP - Vector Red.
In cases where the primary antibody backbone species is the same as the target tissue (for example, using humanized antibodies on human tissue), we prefer to have these antibodies conjugated with a tag (FITC, Biotin, Myc, His), so that an anti-tag antibody can be used for detecting the target. This avoids the problem of detecting endogenous immunoglobulins that are detected by the secondary antibody. Although blocking reagents are used during the immunohistochemistry staining procedure, these reagents can often reduce both background as well as signal, so in our experience, the sensitivity is often better preserved by using a tag rather than targeting the primary antibody Fc fragment of the same species.
When purchasing antibodies or synthesized custom antibodies to a target, the most important factor that determines antibody specificity is the immunogen that was used to generate the antibody. When the antibody targets a specific peptide, the peptide should be unique to the target protein and should not contain regions that are either homologous to other members of its protein family or to other proteins that are highly expressed within a cell. LSBio routinely BLASTs peptide sequences to identify proteins that may cross react with the antibody, and excludes peptide sequences or antibodies that may bind regions that contain those sequences. Even if a peptide appears unique, because the epitope-binding site within tissues is often conformational, antibodies can bind nonspecifically to regions of other proteins that mimic the structure of the peptide antigen. These binding events produce "nonspecific" or "background" staining within tissues that may interfere with pathologic interpretation.
In other cases, and frequently with monoclonal reagents, antibodies have been generated to short stretches of a protein or a full-length protein, in which case specificity must be determined by an independent method, such as a Western blot or testing the antibody in immunohistochemistry on over-expressing or short-term transfected cell lines compared to control cells that lack the gene. It is important to note that these methods provide evidence that the target protein is being detected (sensitivity) but do not prove that another target is not also being detected by the antibody (specificity). This is because Western blots performed by many antibody manufacturers are limited to a small number of over-expressing cell lines, or to one or two tissues that overexpress the protein. Although the gold standard is that the antibody will detect a single band on a Western blot, in LSBio's experience, this occurs most commonly with high copy proteins. We and others have seen many examples of antibodies that perform beautifully and specifically in IHC yet produce weak bands on a tissue Western. Conversely, an antibody that produces a beautiful Western blot band often performs poorly in IHC. Western blots are therefore most useful when they are positive, but less useful as predictors of an antibody's IHC performance when they show weak or multiple bands on cell or tissue extracts.
A more direct test for antibody sensitivity and specificity is to perform IHC or immunocytochemistry (ICC) on over-expressing and negative control cell lines. Short-term transfected cell lines are more frequently positive than stable cell lines in these types of experiments because stable cell lines may express levels of protein that are below the limit of sensitivity of detection for the antibody in IHC. Performing IHC on formalin-fixed, short-term transfected cell lines is also highly predictive of whether or not the reagent will work on formalin-fixed tissues, because the conditions that are used to produce the signal and the fixation condition for the cell lines and the tissues can be experimentally adjusted to parallel one another. These experiments do not address the issue of whether or not the antibody will detect an anomalous protein once it is used on tissues, but they do demonstrate whether or not the antibody is at least detecting the target within cells that are known to express them.
Another assay frequently requested by our customers is the use of peptide or protein to competitively bind the antibody signal in IHC in order to determine if the signal that is detected on a particular cell type can be blocked by the peptide. In our experience, a competitive blocking experiment with a peptide and its antibody can fail to produce useful data on occasion, because many peptides bind nonspecifically to formalin-fixed tissues and can obscure or actually enhance the antibody signal. In LSBio's experience, an IHC blocking experiment is therefore of value only about half the time.
Antigens are preserved in their most natural state in fresh or frozen tissues. Tissue morphology, however, is best preserved by fixing samples in cross-linking or denaturing preservatives such as formalin, paraformaldehyde, alcohol, methyl Carnoy's, picric acid or mercuric-based reagents. After fixation, antigens are often denatured and folded into shapes that are not recognizable by an antibody that has been generated to a native protein. Although steam or microwave-based antigen retrieval methods or the use of Proteinase-K can recover antigenicity in fixed tissues, many antibodies (particularly monoclonal reagents) may not work at all in fixed tissues, even after antigen retrieval.
In our experience, monoclonal antibodies are most frequently positive in tissues that are fresh, frozen, or fixed in acetone for brief periods. With formalin fixation, even after antigen retrieval, we have observed that the majority of commercial mouse monoclonal antibodies will show no signal or a very weak signal in human tissues. Proteinase K can rescue some signals for some antibodies, but about half of these antibodies will still continue to produce a diminished signal compared to frozen tissues or a comparable polyclonal antibody. When monoclonal antibodies do perform well in formalin -fixed tissues, they produce a very low nonspecific background and are often excellent reagents.
Polyclonal antibodies are more frequently positive on fixed tissues (success ranges from 60-75%, depending upon the target class). The drawback of polyclonal antibodies is that they generally produce a higher nonspecific background staining on tissues than monoclonal antibodies, because they detect multiple parts of the immunogen. Frequently observed background reactions include faint nonspecific staining of renal tubules, islets of Langerhans, connective tissue, serum, or smooth muscle. If a polyclonal antibody has a high affinity for its target protein, or the protein is present at a high copy level, the antibody concentration can be reduced to remove or diminish the background staining reaction. Pathologists who have had substantial experience with polyclonal antibodies will often subtract this signal during IHC analysis.
Tissue preserved using fixatives such as paraformaldehyde, methyl Carnoy's, alcohol-based fixatives, or reagents that are advertised specifically for the preservation of mRNA often perform poorly in IHC compared to tissues snap-frozen without any fixation or fixed in neutral buffered formalin.
Prior to testing the antibody, LSBio validates the tissues for preservation of antigenicity after antigen retrieval. Sections from formalin-fixed tissue blocks (multi-tissue or individual tissue) are tested in advance with positive control antibodies that are known to produce a particular strength signal in those tissues. Antibodies that we frequently use for tissue validation include Cytokeratins, Vimentin, CD31 (endothelial marker), CD3 (T Cell marker), CD20 (B cell marker), or GFAP (glial marker for CNS). Tissues are rejected if they do not show the strength of signal that is typical for markers that should be strongly positive in that cell type. The use of positive control antibodies is highly predictive of whether or not a particular tissue will react to a test antibody, and such antibodies are used to validate all tissues blocks that are selected for immunohistochemistry. The same antibodies can also be used for frozen tissues or tissues fixed by any method to determine if the tissue section is showing reactivity with positive control antibodies.
LSBio tests each antibody at a minimum of four dilutions or concentrations on a multi-tissue formalin-fixed, paraffin-embedded tissue array of 22 normal human tissues, and a separate section of 3 brain regions or a multi-cancer array is often included. This fairly comprehensive panel allows the pathologist to create a table of all of the cell types that are positive, the degree to which they are positive on a 0 to 4 scale (0=negative, 1=blush, 2=faint, 3=moderate, 4=strong), a list of tissues and cell types that are negative for staining, and the subcellular or extracellular structure that is showing staining (i.e. cytoplasmic, membranous, nuclear, extracellular, serum, collagen). This analysis provides a comprehensive view of the behavior of the antibody, its sensitivity, its degree of specificity for cell types that are known to be positive, and the level of nonspecific background that the reagent is showing. These results are then compared to known information about the protein within the public domain, or compared to other antibodies to the same target protein. The results allow the pathologist to determine if the antibody is showing staining that is consistent with what is expected for the target. In many cases, the public information on a target is either incomplete, or highly selective (reports on one or two antibodies), but the overall staining pattern for a reagent should be consistent with the literature although any individual antibody is highly likely to show some differences when compared to another antibody to the same target, particularly if the epitope was from a different region of the protein used as the antigen.
Antibodies can fail to perform in IHC for a variety of reasons. If the antibody is too dilute or has a low affinity for its protein, the signal may be falsely negative. If the antibody has not been affinity purified, it may produce a high background on tissues, rendering interpretation difficult. The antibody may detect other proteins besides the target, and produce an anomalous binding pattern in tissues. The antibody may be one of a subset that works best in either frozen or Proteinase-K antigen retrieval rather than steam-based antigen retrieval. Depending upon the initial IHC results, troubleshooting an antibody that is working poorly in IHC is a time-consuming effort, and if other reagents are available to the same target, it may be less expensive and less time-consuming to simply purchase a different product. When we perform contract immunohistochemistry services for our customers, we routinely purchase multiple antibodies to the same target and test them side by side in order to compare the reagents and identify the best performer for a particular study because we have found that it is more cost-effective for our customers.
If an antibody is a particularly valuable reagent, it is possible to improve its signal in IHC by a variety of methods, including changing the antigen retrieval conditions, the pH of the buffer, the exposure time to the primary antibody, or using various blocking methods, but the fundamental distribution of positive staining in tissues is rarely dramatically changed by these experimental procedures. The factors that most dramatically alter an antibody's binding characteristics in IHC are antibody concentration, tissue fixation, and, for fixed tissues, the use of antigen retrieval. If these variables have been tested with the appropriate positive and negative controls, and the antibody continues either to fail to show a signal or shows a high background signal, it is unlikely that manipulating further experimental conditions will improve the signal by very much. At that point, it is time to get another antibody reagent.