Login
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Quick Order
Products
Antibodies
ELISA and Assay Kits
Research Areas
Infectious Disease
Resources
Purchasing
Reference Material
Contact Us
Location
Corporate Headquarters
Vector Laboratories, Inc.
6737 Mowry Ave
Newark, CA 94560
United States
Telephone Numbers
Customer Service: (800) 227-6666 / (650) 697-3600
Contact Us
Additional Contact Details
Login
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Quick Order
Prostate Cancer Immunohistochemistry Markers
Prostate cancer is the most common non-skin cancer in men, and the third leading cause of cancer
death after lung and colorectal cancer. Immunohistochemistry (IHC) is used to facilitate the diagnosis
of prostate carcinoma, to determine whether or not foci are invasive, and to determine if a patient’s
cancer will respond to androgen therapy (Pentyala, 2016).
Early detection of prostate carcinoma relies on both clinical detection (rectal exam or transrectal ultrasound)
and performing serum measurements of proteins such as prostate-specific antigen (PSA / KLK3),
a glycoprotein secreted by epithelial cells of the prostate gland. Prostein is often
used alongside PSA to increase sensitivity in identifying prostate metastases. IHC with antibodies to NKX3-1
are also useful in identifying prostate as a site of potential origin in a metastasis of unknown primary (Kandalaft, 2015). When prostate biopsies are taken,
IHC with markers such as high molecular weight cytokeratins,
p63, EPCAM,
TGFbeta, and
AMACR can determine whether the basal cell myoepithelial layer is intact
or has been infiltrated by the tumor. Furthermore, markers such as GalNac-T3 (GALNT3),
PSMA (FOLH1), hepsin (TMPRSS1),
and PCA3 have been useful to distinguish between prostate cancer and benign prostatic hyperplasia (BPH).
The genetics of prostate cancer:
The progression of genomic alterations that drives prostate cancer involves a number of well described pathways...
Click to Expand Text ▸
Primary IHC Markers In Prostate Cancer
ACPP (PSAP)
ACPP (PSAP) is a dephosphorylating enzyme produced in prostatic glandular epithelium. Increased serum levels of ACPP were shown to be associated with prostate cancer in the 1930’s, particularly in patients with bone metastases. This enzyme was one of the first tumor serum markers, and it was the main prostate cancer biomarker until the discovery of PSA (Gutman, 1938; Muniyan, 2013; Rao, 2008). Higher ACPP expression in tumors has been correlated with increasing tumor stage (Gunia, 2009), although ACPP is thought to be a tumor suppressant by means of vitamin D-associated slowing of prostate growth (Lin, 1992). Lower expression in normal tissue is therefore thought to be a risk factor for the development of cancer (Stewart, 2005; Kong, 2013). Recently, this target has been used successfully to sensitize patient dendritic cells in order to stimulate an immune response to cancer (Fong, 2001). Although it is highly expressed in prostatic glandular epithelium, ACPP is not as specific as PSA, and has been occasionally associated with other tumors such as lung carcinoid tumors (Azumi, 1991).
Staining:
ACPP is expected to have cytoplasmic staining in prostate tissue.
LSBio's recommended antibody to ACPP / PAP for use in immunohistochemistry is ACPP / PAP Antibody LS‑B3108.
Click here for specifications and further information on this antibody.
Click here for specifications and further information on this antibody.
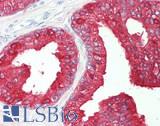
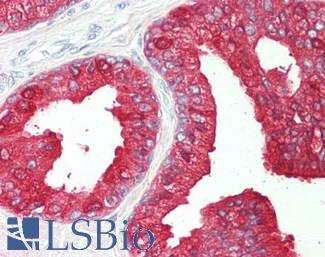
 Anti-Prostatic Acid Phosphatase antibody IHC of human prostate. Immunohistochemistry of formalin-fixed, paraffin-embedded tissue after heat-induced antigen retrieval. Antibody LS-B3108 concentration 5 ug/ml.
Anti-Prostatic Acid Phosphatase antibody IHC of human prostate. Immunohistochemistry of formalin-fixed, paraffin-embedded tissue after heat-induced antigen retrieval. Antibody LS-B3108 concentration 5 ug/ml.
ACPP / PAP Antibody LS-B3108
AMACR
AMACR/P504S is a mitochondrial and peroxisomal enzyme that is overexpressed in prostate intraepithelial neoplasia and prostate cancer (Luo, 2002; Evans, 2003; Lloyd, 2013). AMACR functions to oxidize fatty acids, drugs such as ibuprofen, and bile acid intermediates. AMACR stimulates prostate cancer growth, but unlike other prostate cancer markers, the effects of this target have been demonstrated to be independent of androgen signaling (Zha, 2003). Because of the enzymatic activity of this target, it is a potential mechanistic contributor to the relationship between high consumption of dietary branched chain fatty acids (present in meats and dairy products) and increased risk of prostate cancer (Lloyd, 2008; Zhu, 2005; Stacewicz-Sapuntzakis, 2008; Capurso, 2017).
Staining:
Staining for AMACR is expected to be cytoplasmic.
LSBio's recommended antibody to AMACR / P504S for use in immunohistochemistry is AMACR / P504S Antibody LS‑B3468.
Click here for specifications and further information on this antibody.
Click here for specifications and further information on this antibody.
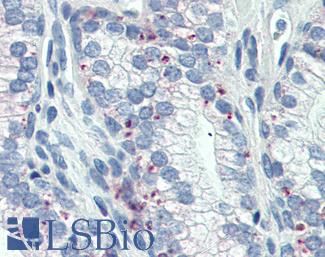
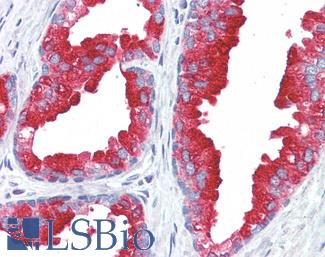
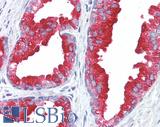
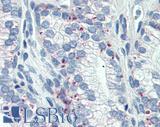 Anti-AMACR antibody IHC of human prostate. Immunohistochemistry of formalin-fixed, paraffin-embedded tissue after heat-induced antigen retrieval. Antibody LS-B3468 dilution 1:200.
Anti-AMACR antibody IHC of human prostate. Immunohistochemistry of formalin-fixed, paraffin-embedded tissue after heat-induced antigen retrieval. Antibody LS-B3468 dilution 1:200.
AMACR / P504S Antibody LS-B3468
CDKN1B
CDKN1B, commonly known as p27 (or KIP1), is an important nuclear regulator of cell cycle progression. p27 protein is specifically involved in g1 arrest, where it functions to stop or slow down the cell division cycle. Loss of p27 nuclear expression and/or translocation from the nucleus to cytoplasm has been associated with poor prognosis and potential disease progression in a variety of cancers, such as squamous carcinomas of head and neck, melanomas, and lung carcinomas (Hnit, 2015; Vallonthaiel, 2016; Dobashi, 2017; Chu, 2008; Denicourt, 2007; Tsihlias, 1998). This target may also be associated with hereditary cancers: mutations in CDKN1B have been linked to an increased risk of prostate cancer (Chang, 2004). Decreased expression of p27 has been associated with growth in pituitary adenomas, and circulating p27 autoantibodies (hence decreased p27 expression) have been correlated with poor prognosis in osteosarcoma (Li, 2016; Martins, 2016; Bamberger, 1999; Teixeira, 2000). Conversely, the indolent course of thyroid papillary carcinomas has been attributed to the presence of p27 (Garcia-Rendueles, 2017). Tumor cells that are quiescent (non-dividing) are protected from the cytotoxicity of many chemotherapeutic agents. Therefore, disrupting the normal nuclear expression of p27, which prevents cells from entering the mitotic pathway, may enhance response rates to chemotherapy, making this target important in the search for new cancer treatments (Becker, 2017; Barzegar, 2017).
Staining:
CDKN1B is expected to have predominantly nuclear staining in normal prostate tissue and may have loss of expression or show cytoplasmic translocation in prostate tumors.
LSBio's recommended antibody to CDKN1B for use in immunohistochemistry is CDKN1B Antibody LS‑B5740.
Click here for specifications and further information on this antibody.
Click here for specifications and further information on this antibody.

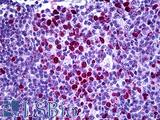 Anti-CDKN1B / p27 Kip1 antibody IHC of human tonsil. Immunohistochemistry of formalin-fixed, paraffin-embedded tissue after heat-induced antigen retrieval. Antibody LS-B5740 concentration 5 ug/ml.
Anti-CDKN1B / p27 Kip1 antibody IHC of human tonsil. Immunohistochemistry of formalin-fixed, paraffin-embedded tissue after heat-induced antigen retrieval. Antibody LS-B5740 concentration 5 ug/ml.
CDKN1B Antibody LS-B5740
ELAC2
ELAC2 (HPC2 / Prostate Cancer, Hereditary, 2) is a nuclear and mitochondrial endonuclease enzyme that functions in tRNA processing (Rossmanith, 2011). Missense variants in ELAC2 are associated with a small percentage of familial prostate cancers (Rebbeck, 2000; Tavtigian, 2000; Xu, 2000; Xu, 2001).
Staining:
ELAC2 may have nuclear or cytoplasmic staining.
LSBio's recommended antibody to ELAC2 for use in immunohistochemistry is ELAC2 Antibody LS‑B5670.
Click here for specifications and further information on this antibody.
Click here for specifications and further information on this antibody.
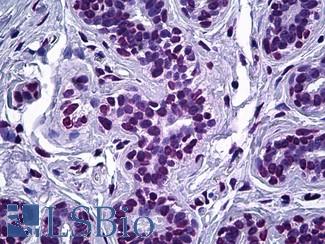
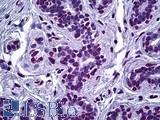 Anti-ELAC2 antibody IHC of human breast, epithelium. Immunohistochemistry of formalin-fixed, paraffin-embedded tissue after heat-induced antigen retrieval. Antibody LS-B5670 dilution 1:100.
Anti-ELAC2 antibody IHC of human breast, epithelium. Immunohistochemistry of formalin-fixed, paraffin-embedded tissue after heat-induced antigen retrieval. Antibody LS-B5670 dilution 1:100.
ELAC2 Antibody LS-B5670
FOLH1 (PSMA)
Folate Hydrolase 1 (FOLH1, also known as Prostate-Specific Membrane Antigen or PSMA) is a membrane-associated protein that is highly expressed in prostatic epithelium. It increases in expression progressively with increasing grade in prostatic intraepithelial neoplasia and prostatic carcinoma. Decreased expression is associated with poor survival in prostate cancer (Bostwick, 1998; Murphy, 1998). The function of FOLH1 is not well understood (Kaittanis, 2018). Recently, this target has been shown to be expressed in neovascular endothelium in a number of non-prostatic carcinomas, including lung, pancreatic, and renal cell carcinomas, and glioblastomas. Positive expression in endothelium may predict a positive response to chemotherapy (Baccala, 2007; Nguyen, 2016; Stock, 2017; Wang, 2015; Wernicke, 2011).
Staining:
FOLH1 is expected to have membranous and cytoplasmic staining in prostate tissue.
LSBio's recommended antibody to FOLH1 / PSMA for use in immunohistochemistry is FOLH1 / PSMA Antibody LS‑B2542.
Click here for specifications and further information on this antibody.
Click here for specifications and further information on this antibody.
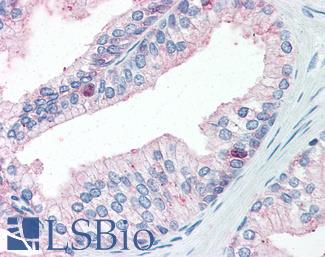
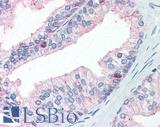 Anti-FOLH1 / PSMA antibody IHC of human prostate. Immunohistochemistry of formalin-fixed, paraffin-embedded tissue after heat-induced antigen retrieval. Antibody LS-B2542 concentration 10 ug/ml.
Anti-FOLH1 / PSMA antibody IHC of human prostate. Immunohistochemistry of formalin-fixed, paraffin-embedded tissue after heat-induced antigen retrieval. Antibody LS-B2542 concentration 10 ug/ml.
FOLH1 / PSMA Antibody LS-B2542
FOXA1
Fork-head box protein A1 is a nuclear transcription factor that is activated in embryogenesis of the liver. It is associated with the function of nuclear hormone receptors including estrogen, and is integral to tamoxifen effectiveness in breast cancer. FOXA1 is also associated with the function and distribution of androgen receptors in prostate tissue, and mutations in FOXA1 have been demonstrated in prostate cancer (Sahu, 2011; Hurtado, 2011; Barbieri, 2012). This target has also been associated with aggressive prostate cancers, and may be an independent predictor of recurrence (Tsourlakis, 2017).
Staining:
FOXA1 is expected to have nuclear expression in lung tissue.
LSBio's recommended antibody to FOXA1 for use in immunohistochemistry is FOXA1 Antibody LS‑B6101.
Click here for specifications and further information on this antibody.
Click here for specifications and further information on this antibody.
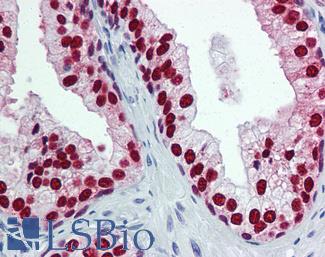
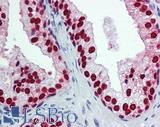 Anti-FOXA1 antibody IHC of human prostate. Immunohistochemistry of formalin-fixed, paraffin-embedded tissue after heat-induced antigen retrieval. Antibody LS-B6101 concentration 5 ug/ml.
Anti-FOXA1 antibody IHC of human prostate. Immunohistochemistry of formalin-fixed, paraffin-embedded tissue after heat-induced antigen retrieval. Antibody LS-B6101 concentration 5 ug/ml.
FOXA1 Antibody LS-B6101
MSMB
Beta-microseminoprotein (MSMB), also known as prostate secretory protein 94 (PSP94), is a secreted immunoglobulin binding protein present in seminal fluid that is synthesized in prostate epithelium (Hara, 1989). The single nucleotide polymorphism rs10993994 in MSMB has been associated with an increased risk for prostate carcinoma (Whitaker, 2010), leading to the theory that MSMB plays a protective role in prevention of prostate carcinoma (Anklesaria, 2018).
Staining:
This protein is expected to have cytoplasmic staining in prostate tissue.
LSBio's recommended antibody to MSMB / MSP for use in immunohistochemistry is MSMB / MSP Antibody LS‑B12982.
Click here for specifications and further information on this antibody.
Click here for specifications and further information on this antibody.
Prostein (SLC45A3 / p501S)
Prostein (also known as prostate cancer-associated protein 6 / P501S / SLC45A3) is a protein present in the golgi apparatus of benign and malignant prostatic glandular epithelium, and shows perinuclear cytoplasmic localization in immunohistochemical experiments (Xu, 2001; Sheridan, 2007). Because it is highly specific for prostate glandular cells, this target is useful for differentiating extra-prostatic metastases from other carcinomas such as urothelial carcinomas or colorectal carcinomas (Xu, 2001; Lane, 2008; Chuang, 2007; Sheridan, 2007). Although it may show diminished expression in some aggressive prostate cancers, this target is sometimes expressed in PSA-negative prostate carcinomas, and these two targets used in combination can lead to increased sensitivity in the identification of prostate cancer metastases (Perner, 2013; Sheridan, 2007).
Staining:
Prostein is expected to have cytoplasmic staining in prostate tissue.
LSBio's recommended antibody to Prostein / SLC45A3 for use in immunohistochemistry is Prostein / SLC45A3 Antibody LS‑B1623.
Click here for specifications and further information on this antibody.
Click here for specifications and further information on this antibody.
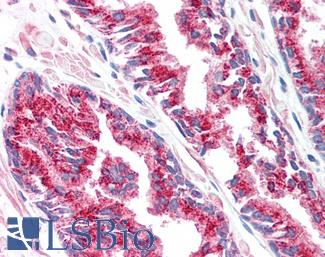
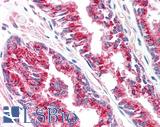 Anti-SLC45A3 / Prostein antibody IHC of human prostate. Immunohistochemistry of formalin-fixed, paraffin-embedded tissue after heat-induced antigen retrieval. Antibody LS-B1623 concentration 5 ug/m
Anti-SLC45A3 / Prostein antibody IHC of human prostate. Immunohistochemistry of formalin-fixed, paraffin-embedded tissue after heat-induced antigen retrieval. Antibody LS-B1623 concentration 5 ug/m
Prostein / SLC45A3 Antibody LS-B1623
Prostate Specific Antigen (KLK3)
Most commonly known as prostate specific antigen (PSA), Kallikrein-Related Peptidase 3 (KLK3) is a secreted protease produced in prostate glandular epithelium that is thought to function in the liquefaction of seminal fluid. PSA is a highly sensitive and specific clinical marker for recurrent prostate carcinoma after prostatectomy (Oesterling, 1991). It is also useful for confirming suspected prostatic origin in the setting of metastatic carcinoma (Stamey, 1987; Oesterling, 1988).
Staining:
PSA is expected to have cytoplasmic staining in prostate tissue.
LSBio's recommended antibody to PSA / KLK3 for use in immunohistochemistry is PSA / KLK3 Antibody LS‑B3470.
Click here for specifications and further information on this antibody.
Click here for specifications and further information on this antibody.
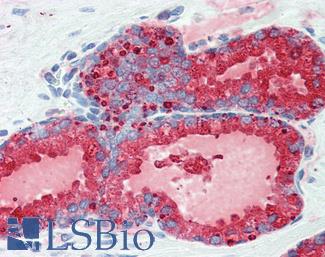
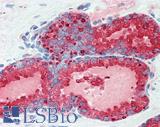 Anti-KLK3 / PSA antibody IHC of human prostate. Immunohistochemistry of formalin-fixed, paraffin-embedded tissue after heat-induced antigen retrieval. Antibody LS-B3470 dilution 1:200.
Anti-KLK3 / PSA antibody IHC of human prostate. Immunohistochemistry of formalin-fixed, paraffin-embedded tissue after heat-induced antigen retrieval. Antibody LS-B3470 dilution 1:200.
PSA / KLK3 Antibody LS-B3470
VEGFA
VEGFA (vascular endothelial growth factor A) is a secreted mitogenic cytokine that stimulates endothelial cells, promoting angiogenesis and increased vascular permeability through interactions with VEGF receptors. VEGFA is the target of antiangiogenic chemotherapueutic agents such as bevacizumab (Grothey, 2008), and measurement of serum VEGFA has been explored as a potential noninvasive means of monitoring efficacy of antiangiogenic therapy in cancers (Caporarello, 2017; Secord, 2014). VEGFA is expressed in osteoblasts and is important in normal bone development. It is thought to play a significant role in the unique propensity for bone involvement of metastatic-prostate cancer, which produces characteristic osteoblastic bone lesions with increased bone density rather than lytic bone lesions associated with other types of cancer (Lee, 2017; Roberts, 2013; Hu, 2016).
Staining:
VEGFA is a secreted cytokine and can show both cytoplasmic and extracellular staining.
LSBio's recommended antibody to VEGFA for use in immunohistochemistry is VEGFA Antibody LS‑B3579.
Click here for specifications and further information on this antibody.
Click here for specifications and further information on this antibody.

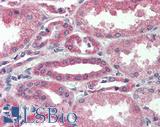 Anti-VEGFA antibody IHC of human kidney. Immunohistochemistry of formalin-fixed, paraffin-embedded tissue after heat-induced antigen retrieval. Antibody LS-B3579 dilution 1:50.
Anti-VEGFA antibody IHC of human kidney. Immunohistochemistry of formalin-fixed, paraffin-embedded tissue after heat-induced antigen retrieval. Antibody LS-B3579 dilution 1:50.
VEGFA Antibody LS-B3579
References ▷
-
Adamo, P. et al. The oncogene ERG: a key factor in prostate cancer. Oncogene 2016-01; 35.4; 403
[Full Text Article] -
Alanee, Shaheen R. et al. Clinical features and management of BRCA1 and BRCA2-associated prostate cancer. Frontiers in Bioscience (Elite Edition) 1/1/2014; 6; 15-30
[Pubmed: 24389137] -
Alumkal, Joshi et al. TMPRSS2-ERG, a Mover and a Shaker. Science Translational Medicine 6/2/2010; 2.34; 34ec86-34ec86
[Full Text Article] -
An, Jian et al. Destruction of full-length androgen receptor by wild-type SPOP, but not prostate-cancer-associated mutants. Cell Reports 2/27/2014; 6.4; 657-669
[Full Text Article] [Pubmed: 24508459] [PMC: PMC4361392] -
Anklesaria, Jenifer H. et al. Structural and molecular biology of PSP94: Its significance in prostate pathophysiology. Frontiers in Bioscience (Landmark Edition) 1/1/2018; 23; 535-562
[Pubmed: 28930560] -
Azumi, N. et al. Prostatic acid phosphatase in carcinoid tumors. Immunohistochemical and immunoblot studies. The American Journal of Surgical Pathology 1991-08; 15.8; 785-790
[Pubmed: 1712549] -
Baccala, Angelo et al. Expression of prostate-specific membrane antigen in tumor-associated neovasculature of renal neoplasms. Urology 2007-08; 70.2; 385-390
[Full Text Article] [Pubmed: 17826525] -
Bamberger, C. M. et al. Reduced expression levels of the cell-cycle inhibitor p27Kip1 in human pituitary adenomas. European Journal of Endocrinology 1999-03; 140.3; 250-255
[Pubmed: 10216521] -
Barbareschi, M. et al. p63, a p53 homologue, is a selective nuclear marker of myoepithelial cells of the human breast. The American Journal of Surgical Pathology 2001-08; 25.8; 1054-1060
[Pubmed: 11474290] -
Barbieri, Christopher E. et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nature genetics 5/20/2012; 44.6; 685-689
[Full Text Article] [Pubmed: 22610119] [PMC: PMC3673022] -
Barzegar, Mansoureh et al. SKLB188 inhibits the growth of head and neck squamous cell carcinoma by suppressing EGFR signalling. British Journal of Cancer 10/10/2017; 117.8; 1154-1163
[Full Text Article] [Pubmed: 28873083] [PMC: PMC5674107] -
Becker, Walter et al. A wake-up call to quiescent cancer cells - potential use of DYRK1B inhibitors in cancer therapy. The FEBS journal 11/28/2017;
[Full Text Article] [Pubmed: 29193696] -
Bostwick, D. G. et al. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer 6/1/1998; 82.11; 2256-2261
[Pubmed: 9610707] -
Boysen, Gunther et al. SPOP mutation leads to genomic instability in prostate cancer. eLife ; 4;
[Full Text Article] [Pubmed: 26374986] [PMC: PMC4621745] -
Caporarello, Nunzia et al. Classical VEGF, Notch and Ang signalling in cancer angiogenesis, alternative approaches and future directions (Review). Molecular Medicine Reports 10/1/2017; 16.4; 4393-4402
-
Capurso, Cristiano et al. The Mediterranean Diet Reduces the Risk and Mortality of the Prostate Cancer: A Narrative Review. Frontiers in Nutrition 2017; 4; 38
[Full Text Article] [Pubmed: 28884114] [PMC: PMC5573712] -
Castro, Elena et al. Effect of BRCA Mutations on Metastatic Relapse and Cause-specific Survival After Radical Treatment for Localised Prostate Cancer. European Urology 2015-08; 68.2; 186-193
[Full Text Article] [Pubmed: 25454609] -
Chang, Bao-li et al. A polymorphism in the CDKN1B gene is associated with increased risk of hereditary prostate cancer. Cancer Research 3/15/2004; 64.6; 1997-1999
[Pubmed: 15026335] -
Chu, Isabel M. et al. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nature Reviews. Cancer 2008-04; 8.4; 253-267
[Full Text Article] [Pubmed: 18354415] -
Chuang, Ai-Ying et al. Immunohistochemical differentiation of high-grade prostate carcinoma from urothelial carcinoma. The American Journal of Surgical Pathology 2007-08; 31.8; 1246-1255
[Full Text Article] [Pubmed: 17667550] -
Dhingra, Priyanka et al. Identification of novel prostate cancer drivers using RegNetDriver: a framework for integration of genetic and epigenetic alterations with tissue-specific regulatory network. Genome Biology 7/27/2017; 18; 141
[Full Text Article] -
Dobashi, Yoh et al. Regulation of p27 by ubiquitin ligases and its pathological significance in human lung carcinomas. Human Pathology 2017; 66; 67-78
[Full Text Article] [Pubmed: 28601655] -
Edwards, S. et al. Expression analysis onto microarrays of randomly selected cDNA clones highlights HOXB13 as a marker of human prostate cancer. British Journal of Cancer 1/31/2005; 92.2; 376-381
[Full Text Article] [Pubmed: 15583692] [PMC: PMC2361840] -
Ewing, Charles M. et al. Germline mutations in HOXB13 and prostate-cancer risk. The New England Journal of Medicine 1/12/2012; 366.2; 141-149
[Full Text Article] [Pubmed: 22236224] [PMC: PMC3779870] -
Fong, L. et al. Dendritic cell-based xenoantigen vaccination for prostate cancer immunotherapy. Journal of Immunology (Baltimore, Md.: 1950) 12/15/2001; 167.12; 7150-7156
[Pubmed: 11739538] -
Garcia-Rendueles, A. R. et al. Rewiring of the apoptotic TGF-β-SMAD/NFκB pathway through an oncogenic function of p27 in human papillary thyroid cancer. Oncogene 2/2/2017; 36.5; 652-666
[Full Text Article] [Pubmed: 27452523] -
Gimm, Oliver et al. Differential Nuclear and Cytoplasmic Expression of PTEN in Normal Thyroid Tissue, and Benign and Malignant Epithelial Thyroid Tumors. The American Journal of Pathology 2000-05; 156.5; 1693-1700
[Pubmed: 10793080] [PMC: PMC1876937] -
Grothey, Axel et al. Targeting angiogenesis driven by vascular endothelial growth factors using antibody-based therapies. Cancer Journal (Sudbury, Mass.) 2008-06; 14.3; 170-177
[Full Text Article] [Pubmed: 18536556] -
Gunia, Sven et al. Expression of prostatic acid phosphatase (PSAP) in transurethral resection specimens of the prostate is predictive of histopathologic tumor stage in subsequent radical prostatectomies. Virchows Archiv: An International Journal of Pathology 2009-05; 454.5; 573-579
[Full Text Article] [Pubmed: 19301031] -
Gutman, Alexander B. et al. AN “ ACID ” PHOSPHATASE OCCURRING IN THE SERUM OF PATIENTS WITH METASTASIZING CARCINOMA OF THE PROSTATE GLAND. Journal of Clinical Investigation 1938-07; 17.4; 473-478
[Pubmed: 16694594] [PMC: PMC434803] -
Hara, M. et al. Two prostate-specific antigens, gamma-seminoprotein and beta-microseminoprotein. The Journal of Laboratory and Clinical Medicine 1989-05; 113.5; 541-548
[Pubmed: 2654306] -
Hnit, Su Su Thae et al. p27(Kip1) signaling: Transcriptional and post-translational regulation. The International Journal of Biochemistry & Cell Biology 2015-11; 68; 43357
[Full Text Article] [Pubmed: 26279144] -
Hu, Kai et al. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. The Journal of Clinical Investigation ; 126.2; 509-526
[Full Text Article] [Pubmed: 26731472] [PMC: PMC4731163] -
Huang, Liwei et al. Posterior Hox gene expression and differential androgen regulation in the developing and adult rat prostate lobes. Endocrinology 2007-03; 148.3; 1235-1245
[Full Text Article] [Pubmed: 17138648] [PMC: PMC2276874] -
Hurtado, Antoni et al. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nature Genetics 2011-01; 43.1; 27-33
[Full Text Article] [Pubmed: 21151129] [PMC: PMC3024537] -
Jung, Chaeyong et al. HOXB13 induces growth suppression of prostate cancer cells as a repressor of hormone-activated androgen receptor signaling. Cancer Research 12/15/2004; 64.24; 9185-9192
[Full Text Article] [Pubmed: 15604291] -
Kandalaft, Patricia L. et al. Practical Applications in Immunohistochemistry: Carcinomas of Unknown Primary Site. Archives of Pathology & Laboratory Medicine 2016-06; 140.6; 508-523
[Full Text Article] [Pubmed: 26457625] -
Kong, Hoon Young et al. Emerging Roles of Human Prostatic Acid Phosphatase. Biomolecules & Therapeutics 2013-01; 21.1; 43393
[Full Text Article] [Pubmed: 24009853] [PMC: PMC3762301] -
Kristiansen, Ilka et al. Sensitivity of HOXB13 as a Diagnostic Immunohistochemical Marker of Prostatic Origin in Prostate Cancer Metastases: Comparison to PSA, Prostein, Androgen Receptor, ERG, NKX3.1, PSAP, and PSMA. International Journal of Molecular Sciences 5/29/2017; 18.6; 1151
[Full Text Article] -
Kwon, Jeong Eun et al. BTB domain-containing speckle-type POZ protein (SPOP) serves as an adaptor of Daxx for ubiquitination by Cul3-based ubiquitin ligase. The Journal of Biological Chemistry 5/5/2006; 281.18; 12664-12672
[Full Text Article] [Pubmed: 16524876] -
Lane, Zhaoli et al. Immunohistochemical expression of prostatic antigens in adenocarcinoma and villous adenoma of the urinary bladder. The American Journal of Surgical Pathology 2008-09; 32.9; 1322-1326
[Full Text Article] [Pubmed: 18670358] -
Lee, Changki et al. Dual targeting c-met and VEGFR2 in osteoblasts suppresses growth and osteolysis of prostate cancer bone metastasis. Cancer Letters 2/1/2018; 414; 205-213
[Full Text Article] [Pubmed: 29174801] -
Li, J. et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science (New York, N.Y.) 3/28/1997; 275.5308; 1943-1947
[Pubmed: 9072974] -
Li, Yiting et al. p27 Is a Candidate Prognostic Biomarker and Metastatic Promoter in Osteosarcoma. Cancer Research 2016; 76.13; 4002-4011
[Full Text Article] [Pubmed: 27197201] [PMC: PMC4930684] -
Lin, M. F. et al. Expression of human prostatic acid phosphatase activity and the growth of prostate carcinoma cells. Cancer Research 9/1/1992; 52.17; 4600-4607
[Pubmed: 1380886] -
Lloyd, Matthew D. et al. Alpha-methylacyl-CoA racemase--an 'obscure' metabolic enzyme takes centre stage. The FEBS journal 2008-03; 275.6; 1089-1102
[Full Text Article] [Pubmed: 18279392] -
Lloyd, Matthew D. et al. α-Methylacyl-CoA racemase (AMACR): metabolic enzyme, drug metabolizer and cancer marker P504S. Progress in Lipid Research 2013-04; 52.2; 220-230
[Full Text Article] [Pubmed: 23376124] -
Luo, Jun et al. Alpha-methylacyl-CoA racemase: a new molecular marker for prostate cancer. Cancer Research 4/15/2002; 62.8; 2220-2226
[Pubmed: 11956072] -
Martins, C. S. et al. P27/CDKN1B Translational Regulators in Pituitary Tumorigenesis. Hormone and Metabolic Research = Hormon- Und Stoffwechselforschung = Hormones Et Metabolisme 2016-12; 48.12; 840-846
[Full Text Article] [Pubmed: 27824399] -
Milella, Michele et al. PTEN: Multiple Functions in Human Malignant Tumors. Frontiers in Oncology 2/16/2015; 5;
[Full Text Article] [Pubmed: 25763354] [PMC: PMC4329810] -
Muniyan, Sakthivel et al. Human Prostatic Acid Phosphatase: Structure, Function and Regulation. International Journal of Molecular Sciences 5/21/2013; 14.5; 10438-10464
[Full Text Article] [Pubmed: 23698773] [PMC: PMC3676848] -
Murphy, G. P. et al. Current evaluation of the tissue localization and diagnostic utility of prostate specific membrane antigen. Cancer 12/1/1998; 83.11; 2259-2269
[Pubmed: 9840525] -
Nagai, Y. et al. Identification of a novel nuclear speckle-type protein, SPOP. FEBS letters 11/24/1997; 418.43102; 23-26
[Pubmed: 9414087] -
Nguyen, Daniel P. et al. Induction of PSMA and Internalization of an Anti-PSMA mAb in the Vascular Compartment. Molecular cancer research: MCR 2016; 14.11; 1045-1053
[Full Text Article] [Pubmed: 27458033] -
Norris, John D. et al. The Homeodomain Protein HOXB13 Regulates the Cellular Response to Androgens. Molecular Cell 11/13/2009; 36.3; 405-416
[Full Text Article] [Pubmed: 19917249] -
Oesterling, J. E. et al. Prostate specific antigen: a critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. The Journal of Urology 1991-05; 145.5; 907-923
[Pubmed: 1707989] -
Paramio, J. M. et al. PTEN tumour suppressor is linked to the cell cycle control through the retinoblastoma protein. Oncogene 12/9/1999; 18.52; 7462-7468
[Full Text Article] [Pubmed: 10602505] -
Pentyala, Srinivas et al. Prostate cancer markers: An update. Biomedical Reports 2016-03; 4.3; 263-268
[Full Text Article] [Pubmed: 26998261] [PMC: PMC4774403] -
Perner, Sven et al. Loss of SLC45A3 protein (prostein) expression in prostate cancer is associated with SLC45A3-ERG gene rearrangement and an unfavorable clinical course. International Journal of Cancer 2/15/2013; 132.4; 807-812
[Full Text Article] [Pubmed: 22821757] -
Petrucelli, Nancie et al. BRCA1- andBRCA2-Associated Hereditary Breast and Ovarian Cancer. GeneReviews® 1993;
[Pubmed: 20301425] -
Phin, Sopheap et al. Genomic Rearrangements of PTEN in Prostate Cancer. Frontiers in Oncology 9/17/2013; 3; 240
[Full Text Article] [Pubmed: 24062990] [PMC: PMC3775430] -
Planchon, Sarah M. et al. The nuclear affairs of PTEN. Journal of Cell Science 2/1/2008; 121.Pt 3; 249-253
[Full Text Article] [Pubmed: 18216329] -
Quinn, D. I. et al. Molecular markers of prostate cancer outcome. European journal of cancer (Oxford, England : 1990) 2005-04; 41.6; 858-887
[Full Text Article] [Pubmed: 15808955] -
Rao, Amrith Raj et al. The discovery of prostate-specific antigen. BJU international 2008-01; 101.1; 43230
[Full Text Article] [Pubmed: 17760888] -
Rebbeck, Timothy R. et al. Association of HPC2/ELAC2 Genotypes and Prostate Cancer. The American Journal of Human Genetics 10/1/2000; 67.4; 1014-1019
[Full Text Article] [Pubmed: 10986046] -
Roberts, Emma et al. The Role of Vascular Endothelial Growth Factor in Metastatic Prostate Cancer to the Skeleton. Prostate Cancer 2013; 2013;
[Full Text Article] [Pubmed: 24396604] [PMC: PMC3874956] -
Robinson, Dan et al. Integrative clinical genomics of advanced prostate cancer. Cell 5/21/2015; 161.5; 1215-1228
[Full Text Article] [Pubmed: 26000489] [PMC: PMC4484602] -
Rossmanith, Walter et al. Localization of human RNase Z isoforms: dual nuclear/mitochondrial targeting of the ELAC2 gene product by alternative translation initiation. PloS One 4/29/2011; 6.4; e19152
[Full Text Article] [Pubmed: 21559454] [PMC: PMC3084753] -
Sahu, Biswajyoti et al. Dual role of FoxA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer. The EMBO journal 9/13/2011; 30.19; 3962-3976
[Full Text Article] [Pubmed: 21915096] [PMC: PMC3209787] -
Schultz, Kris Ann P. et al. PTEN, DICER1, FH , and Their Associated Tumor Susceptibility Syndromes: Clinical Features, Genetics, and Surveillance Recommendations in Childhood. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research 6/15/2017; 23.12; e76-e82
[Full Text Article] [Pubmed: 28620008] -
Secord, Angeles Alvarez et al. The search for biomarkers to direct antiangiogenic treatment in epithelial ovarian cancer. Gynecologic Oncology 2014-11; 135.2; 349-358
[Full Text Article] [Pubmed: 25178997] -
Sheridan, Todd et al. The role of P501S and PSA in the diagnosis of metastatic adenocarcinoma of the prostate. The American Journal of Surgical Pathology 2007-09; 31.9; 1351-1355
[Full Text Article] [Pubmed: 17721190] -
Shtivelman, Emma et al. Molecular pathways and targets in prostate cancer. Oncotarget 8/29/2014; 5.17; 7217-7259
[Pubmed: 25277175] [PMC: PMC4202120] -
Signoretti, S. et al. p63 is a prostate basal cell marker and is required for prostate development. The American Journal of Pathology 2000-12; 157.6; 1769-1775
[Full Text Article] [Pubmed: 11106548] [PMC: PMC1885786] -
Stacewicz-Sapuntzakis, Maria et al. Correlations of dietary patterns with prostate health. Molecular Nutrition & Food Research 2008-01; 52.1; 114-130
[Full Text Article] [Pubmed: 18080240] -
Stamey, Thomas A. et al. Prostate-Specific Antigen as a Serum Marker for Adenocarcinoma of the Prostate. New England Journal of Medicine 10/8/1987; 317.15; 909-916
[Full Text Article] [Pubmed: 2442609] -
Stewart, LaMonica V. et al. Vitamin D receptor agonists induce prostatic acid phosphatase to reduce cell growth and HER-2 signaling in LNCaP-derived human prostate cancer cells. The Journal of Steroid Biochemistry and Molecular Biology 2005-10; 97.43102; 37-46
[Full Text Article] [Pubmed: 16076555] -
Stock, Katharina et al. Neovascular Prostate-Specific Membrane Antigen Expression Is Associated with Improved Overall Survival under Palliative Chemotherapy in Patients with Pancreatic Ductal Adenocarcinoma. BioMed Research International 2017; 2017; 2847303
[Full Text Article] [Pubmed: 29209626] [PMC: PMC5676347] -
Tavtigian, Sean V. et al. A candidate prostate cancer susceptibility gene at chromosome 17p. Nature Genetics 2001-02; 27.2; 172
[Full Text Article] -
Taylor, Renea A. et al. Germline BRCA2 mutations drive prostate cancers with distinct evolutionary trajectories. Nature Communications 1/9/2017; 8; 13671
[Full Text Article] -
Teixeira, L. T. et al. p27Kip1-deficient mice exhibit accelerated growth hormone-releasing hormone (GHRH)-induced somatotrope proliferation and adenoma formation. Oncogene 4/6/2000; 19.15; 1875-1884
[Full Text Article] [Pubmed: 10773877] -
Theodorou, Elias et al. A high throughput embryonic stem cell screen identifies Oct-2 as a bifunctional regulator of neuronal differentiation. Genes & Development 3/1/2009; 23.5; 575-588
[Full Text Article] [Pubmed: 19270158] [PMC: PMC2658525] -
Theurillat, Jean-Philippe P. et al. Prostate cancer. Ubiquitylome analysis identifies dysregulation of effector substrates in SPOP-mutant prostate cancer. Science (New York, N.Y.) 10/3/2014; 346.6205; 85-89
[Full Text Article] [Pubmed: 25278611] [PMC: PMC4257137] -
Tomlins, Scott A. et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science (New York, N.Y.) 10/28/2005; 310.5748; 644-648
[Full Text Article] [Pubmed: 16254181] -
Tsihlias, J. et al. Loss of cyclin-dependent kinase inhibitor p27Kip1 is a novel prognostic factor in localized human prostate adenocarcinoma. Cancer Research 2/1/1998; 58.3; 542-548
[Pubmed: 9458103] -
Vallonthaiel, Archana George et al. Prognostic significance of cytoplasmic p27 in oral squamous cell carcinoma. Journal of Oral Pathology & Medicine: Official Publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology 2016-08; 45.7; 475-480
[Full Text Article] [Pubmed: 26750594] -
Wang, Hai-long et al. Expression of prostate-specific membrane antigen in lung cancer cells and tumor neovasculature endothelial cells and its clinical significance. PloS One 2015; 10.5; e0125924
[Full Text Article] [Pubmed: 25978404] [PMC: PMC4433228] -
Wernicke, A. Gabriella et al. Prostate-specific membrane antigen as a potential novel vascular target for treatment of glioblastoma multiforme. Archives of Pathology & Laboratory Medicine 2011-11; 135.11; 1486-1489
[Full Text Article] [Pubmed: 22032578] -
Whitaker, Hayley C. et al. The rs10993994 Risk Allele for Prostate Cancer Results in Clinically Relevant Changes in Microseminoprotein-Beta Expression in Tissue and Urine. PLoS ONE 10/13/2010; 5.10;
[Full Text Article] [Pubmed: 20967219] [PMC: PMC2954177] -
Xu, J. et al. Combined analysis of hereditary prostate cancer linkage to 1q24-25: results from 772 hereditary prostate cancer families from the International Consortium for Prostate Cancer Genetics. American Journal of Human Genetics 2000-03; 66.3; 945-957
[Pubmed: 10712209] [PMC: PMC1288175] -
Xu, Jiangchun et al. Identification and Characterization of Prostein, a Novel Prostate-specific Protein. Cancer Research 2/2/2001; 61.4; 1563-1568
[Pubmed: 11245466] -
Zha, Shan et al. Alpha-methylacyl-CoA racemase as an androgen-independent growth modifier in prostate cancer. Cancer Research 11/1/2003; 63.21; 7365-7376
[Pubmed: 14612535] -
Zhou, Ye et al. Androgens and androgen receptor signaling in prostate tumorigenesis. Journal of Molecular Endocrinology 2/1/2015; 54.1; R15-R29
[Full Text Article] [Pubmed: 25351819]