order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Location
Corporate Headquarters
Vector Laboratories, Inc.
6737 Mowry Ave
Newark, CA 94560
United States
Telephone Numbers
Customer Service: (800) 227-6666 / (650) 697-3600
Contact Us
Additional Contact Details
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Paramyxoviridae (Mumps)
Paramyxoviruses are negative-sense single stranded RNA viruses that cause a wide variety of diseases in human and animals. The majority of paramyxoviruses are respiratory pathogens and transmission occurs via aerosols or direct contact with infectious secretions, or in some cases, by fecal-oral contact.
About Paramyxoviridae
Paramyxoviridae contain two subfamilies: Paramyxovirinae and Pneumovirinae. The Paramyxovirinae subfamily contains five genera: Respirovirus (human parainfluenza virus 3, Sendai), Rubulavirus (Mumps, human parainfluenza virus 5), Avulavirus (Avian parainfluenza, Newcastle disease), Morbillivirus (Measles, canine distemper), and Henipavirus. The second subfamily, Pneumovirinae contains Pneumovirus and metapneumovirus as well as animal pathogens such as human and bovine respiratory syncytial viruses, and human and avian metapneumovirus.
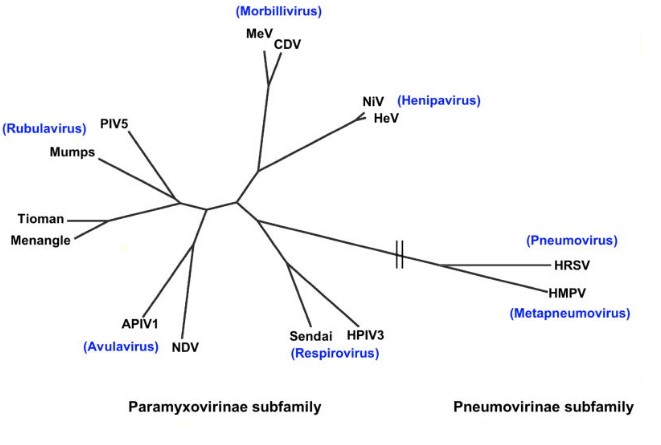
Figure 1: Phylogeny of Paramyxoviridae (Aguilar and Lee, 2011). Legend: APIV1, avian parainfluenza virus 1; CDV, canine distemper virus; HeV, Hendra virus; HMPV, human metapneumovirus; HPIV3, human parainfluenzavirus3; HRSV, human respiratory syncytial virus; MeV, measles virus; NDV, Newcastle disease virus; NiV, Nipah virus; PIV-5, parainfluenzavirus 5.
Parainfluenza viruses (HPIV) are divided into four groups (Types 1-4), and are all enveloped, negative-sense RNA genomes that cause lower respiratory infections in children, the immunocompromised, and the elderly. These viruses also cause significant veterinary disease. There are two genera of HPIV, the Respirovirus (HPIV-1 and HPIV-3) and Rubulavirus (HPIV-2 and HPIV-4). Both differ from influenza viruses by their non-segmented genomes. They also differ from other genera of Paramyxoviridae by the absence of a neuraminidase or nucleocapsid morphology. These viruses are between 150 and 250 nm, and contain a 15,000 nucleotide (-)ssRNA genome that encodes six common structural proteins (N, P, C, M, F, HN, L). Two surface glycoproteins are found in all HPIV: the hemagglutinin-neuraminidase (HN), and the fusion protein (Fo). Beneath the viral membrane is the M protein (membrane). The P gene produces several nonstructural proteins from overlapping reading frames that aid in viral replication or slowing the cell cycle. The V and C proteins may also be involved in inhibition of the interferon response by inducing degradation of STAT1 or STAT2. The nucleocapsid is comprised of viral RNA together with the N, P, and L proteins. The surface glycoproteins HN and F interact with the M protein to direct insertion and aggregation at the cell membrane, with participation of the M protein in cell budding.
The virus first enters the cell by fusing the viral and host cell lipid membranes, followed by release of the HPIV nucleocapsid into the cytoplasm. Replication than takes place by using viral RNA-dependent RNA polymerase (L protein). The cellular ribosomal machinery translates viral mRNA into poroteins, which then direct the full-length replication of the viral genome, first into (+)-sense RNA, then into the (-)-sense RNA strand. These (-)ssRNA starnds area then encapsdated with NP and packaged for export as a new virion.
HPIV can infect many different animals, including birds, primates and rodents. Some parainfluenzaviruses can infect poultry and penguins (Newcastle disease virus).
Clinical features of HPIV infection include both upper and lower respiratory tract illneses. Children present with fever, croup, laryngeal obstruction, and inspiratory stridor. The virus can also cause bonchiolitis in infants, pneumonia in children, tracheobronchitis, and giant-cell pneumonia in immunocompromised individuals. It has been associated with neurologic disease including seizures, encephalitis, and demyelinating syndromes, suggesting that the virus can be neurotropic.
Other important disease causing members of the Rubivirus family is the Mumps virus (MuV), which causes parotitis and orchitis and is also highly neurotropic and may cause encephalitis and meningitis. Although the disease is self-limiting and rarely fatal, long germ sequelae can occur, including seizures, deafness, and paralysis. The virus is transmitted by inhalation or oral contact with droplets, and the hallmark is salivary gland swelling, with viral replication occurring in the parotid glands bilaterally. Unilateral orchitis is the most common extra-salivary organ infected by mumps virus. Atrophy of the testicle can occur in half of cases, but sterility is a rare complication. The virus can also infect the kidneys and cause nephritis, as well as pancreatitis in a small percentage of cases. Half of cases of infection show virus within the cerebrospinal fluid and infect neurons in the brain, but mengingitis and encephalitis are rare complications. Deafness can also occur as a consequence of infection within the inner ear.
Henipavirus and Nipahvirus have broad tropism and extreme virulence and infect bats which can transmit to humans either directly or via an intermediate host. Due to their high virulence, they are classified as BSL4 pathogens.
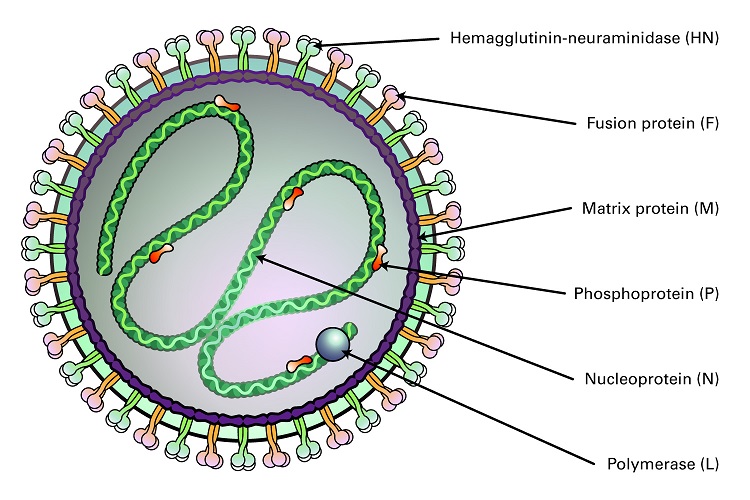
Figure 2: Structure of Paramyxoviridae Virus
References
- Aguilar, HC and Lee, B. Emerging paramyxoviruses: molecular mechanisms and antiviral strategist. 2011. Expert Rev Mol Med. Feb24:13:36. doi: 10.1017/S1462399410001754
- Cox, RM and Plemper, RK. Structure and organization of paramyxovirus particles. 2017. Curr Opin Virol Jun 24:105-114. doi: 10.1016/j.coviro.2017.05.004
- Henrickson, KJ. Parainfluenza Viruses. 2003. Clin Microbiol Reviews. doi: 10.1128/CMR.16.2.242-264.2003
- Rubin, S. et al. Molecular biology, pathogenesis and pathology of mumps virus. 2016 J. Pathol 235(2):242-252. doi: 10.1002/path.4445
- Zeltina, A et al. Emerging Paramyxoviruses: Receptor Tropism and Zoonotic Potential. 2016. PLOS Pathogens. doi: 10.1371/journal.ppat.1005390










