Login
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Quick Order
Products
Antibodies
ELISA and Assay Kits
Research Areas
Infectious Disease
Resources
Purchasing
Reference Material
Contact Us
Location
Corporate Headquarters
Vector Laboratories, Inc.
6737 Mowry Ave
Newark, CA 94560
United States
Telephone Numbers
Customer Service: (800) 227-6666 / (650) 697-3600
Contact Us
Additional Contact Details
Login
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Quick Order
PathPlusTM Osteonectin / SPARC Antibodies
SPARC (secreted protein acidic and rich in cysteine, Osteonectin, BM-40) is a matricellular glycoprotein that modulates cellular interactions with the ECM and is expressed in tissues undergoing remodeling. It functions as a de-adhesive protein, as a modulator of growth factor activity, and as a cell-cycle inhibitor. It induces changes in endothelial cell shape via F-actin, coincident with the appearance of intercellular gaps, that provide a paracellular pathway for extravasation of macromolecules. Tumor growth is enhanced in mice lacking SPARC due to changes in the ECM that create a more permissive environment for tumor progression. Additionally, SPARC is overexpressed in breast, prostate, colorectal and other cancers, and high expression of SPARC is a marker of poor prognosis in rectal cancer. In immunohistochemistry of normal tissue, SPARC has cytoplasmic positivity in endothelial cells, megakaryocytes, fibroblasts, some glial cells, seminiferous ducts of the testes, and neurons in the brain.
References: The UniProt Consortium. Nucleic Acids Res. 47: D506-515 (2019); Nucleic Acids Res. 2016 Jan 4;44(D1):D733-45, PMID:26553804; Cancer Biomark. 2017;18(4):459-466, PMID: 28009327;
2 PathPlusTM Antibodies
☰ Filters
Products
Antibodies
(2)
Type
Primary
(2)
Target
Osteonectin / SPARC
(2)
Reactivity
Human
(1)
Application
IHC-P
(2)
WB
(1)
Host
rabbit
(1)
mouse
(1)
Product Group
PathPlus Cancer
(2)
Isotype
IgG1
(1)
Clonality
monoclonal mc
(1)
polyclonal pc
(1)
Clone
2B1
(1)
Format
Unconjugated
(2)
Publications
No
(2)

Cancer
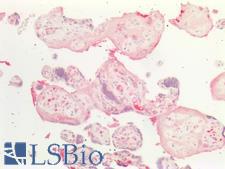
Osteonectin / SPARC Mouse anti-Human Monoclonal (2B1) Antibody
Human
IHC-P, WB
Unconjugated
50 µl/$375

Cancer
Fast Shipping
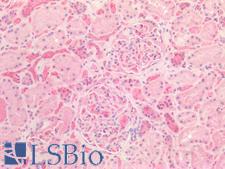
Osteonectin / SPARC Rabbit anti-Human Polyclonal Antibody
IHC-P
Unconjugated
50 µg/$395
Viewing 1-2
of 2
product results











