Login
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Quick Order
Products
Antibodies
ELISA and Assay Kits
Research Areas
Infectious Disease
Resources
Purchasing
Reference Material
Contact Us
Location
Corporate Headquarters
Vector Laboratories, Inc.
6737 Mowry Ave
Newark, CA 94560
United States
Telephone Numbers
Customer Service: (800) 227-6666 / (650) 697-3600
Contact Us
Additional Contact Details
Login
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Quick Order
PathPlusTM SDHB Antibodies
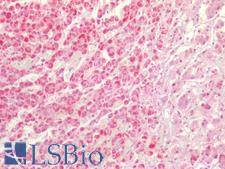
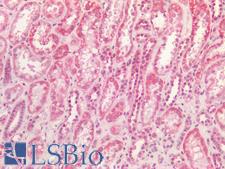
SDHB (Succinate dehydrogenase [ubiquinone] iron-sulfur subunit, mitochondrial) is an iron-sulfur protein subunit of complex II, having an intermediate role in the electron transfer from the citric acid cycle to the respiratory chain. The complex is composed of four nuclear-encoded subunits and is localized in the mitochondrial inner membrane. The iron-sulfur subunit is highly conserved and contains three cysteine-rich clusters which may comprise the iron-sulfur centers of the enzyme. Sporadic and familial mutations in this gene result in paragangliomas and pheochromocytoma, and support a link between mitochondrial dysfunction and tumorigenesis. In immunohistochemistry of normal tissue, SDHB has cytoplasmic positivity in all cell types in tissues throughout the body.
References: The UniProt Consortium. Nucleic Acids Res. 47: D506-515 (2019); Nucleic Acids Res. 2016 Jan 4;44(D1):D733-45, PMID:26553804; J. Clin. Endocrinol. Metab. 91 (11): 4505–9, PMID: 16912137;
2 PathPlusTM Antibodies
☰ Filters
Products
Antibodies
(2)
Type
Primary
(2)
Target
SDHB
(2)
Reactivity
Human
(2)
Mouse
(1)
Application
IHC-P
(2)
WB
(2)
ELISA
(1)
ICC
(1)
IF
(1)
Peptide-ELISA
(1)
Host
rabbit
(1)
goat
(1)
Product Group
PathPlus Cancer
(2)
Clonality
polyclonal pc
(2)
Format
Unconjugated
(2)
Epitope
aa1-150
(1)
aa271-280
(1)
Publications
No
(1)
Yes
(1)

Cancer
SDHB Rabbit anti-Human Polyclonal (aa1-150) Antibody
Mouse, Human
ICC, IF, IHC-P, WB
Unconjugated
0.1 ml/$375

Cancer
SDHB Goat anti-Human Polyclonal (aa271-280) Antibody
Human
ELISA, IHC-P, Peptide-ELISA, WB
Unconjugated
50 µg/$375
Viewing 1-2
of 2
product results











