Login
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Quick Order
Products
Antibodies
ELISA and Assay Kits
Research Areas
Infectious Disease
Resources
Purchasing
Reference Material
Contact Us
Location
Corporate Headquarters
Vector Laboratories, Inc.
6737 Mowry Ave
Newark, CA 94560
United States
Telephone Numbers
Customer Service: (800) 227-6666 / (650) 697-3600
Contact Us
Additional Contact Details
Login
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Forgot password?
Registration enables users to use special features of this website, such as past
order histories, retained contact details for faster checkout, review submissions, and special promotions.
order histories, retained contact details for faster checkout, review submissions, and special promotions.
Quick Order
| Catalog Number | Size | Price |
|---|---|---|
| LS-B16430-50 | 50 µg | $460 |



1 of 3
2 of 3
3 of 3
PathPlus™ Monoclonal Mouse anti‑Human HSPB1 / HSP27 Antibody (IHC, WB) LS‑B16430
PathPlus™ Monoclonal Mouse anti‑Human HSPB1 / HSP27 Antibody (IHC, WB) LS‑B16430
Note: This antibody replaces LS-C26684
Antibody:
HSPB1 / HSP27 Mouse anti-Human Monoclonal Antibody
Application:
IHC-P, IHC-Fr, WB, IP, ELISA
Reactivity:
Human
Format:
Unconjugated, Unmodified
Toll Free North America
 (800) 227-6666
(800) 227-6666
For Research Use Only
Overview
Antibody:
HSPB1 / HSP27 Mouse anti-Human Monoclonal Antibody
Application:
IHC-P, IHC-Fr, WB, IP, ELISA
Reactivity:
Human
Format:
Unconjugated, Unmodified
Specifications
Description
HSPB1 (heat shock protein beta-1, HSP27, HSP28, CMT2F, Hsp25) is a chaperone protein of the sHsp (small heat shock protein) family that protects against oxidative stress. It is overexpressed during oxidative stress to reduce BAX activation and H2O2-induced autophagy and apoptosis within the injured cell. HSPB1 translocates from the cytoplasm to the nucleus upon stress induction. It is generally involved in chaperone activity, thermotolerance, cell survival and inhibition of apoptosis, regulation of cell development and differentiation, and signal transduction. It is overexpressed in various cancers, including hepatocellular, gastric, colorectal, lung and breast carcinomas, and it is associated with a poor prognosis by protecting cells from agents that normally induce apoptosis. Mutated HSPB1 is also causative for Charcot-Marie-Tooth neuropathy and is associated with distal hereditary motor neuropathy. Alternatively, upregulation of wild-type HSPB1 is thought to protect against some of the negative effects of neurodegenerative diseases including Alzheimer’s and Parkinson’s disease. In normal tissues, it is expressed in the cytoplasm with some nuclear positivity in muscle cells and squamous epithelial cells throughout the body.
References: Cellular Signalling. 2014. 26 (7): 1616–25, PMID: 24686082; International Journal of Clinical Chemistry. 2013. 417: 73–9, PMID: 23266770; Cellular and Molecular Life Sciences. 2005. 62 (6): 670–84, PMID: 15770419; Journal of Neuroscience. 2011. 31 (43) 15320-15328; DOI: 10.1523/JNEUROSCI.3266-11.2011; PlosOne 2015 doi.org/10.1371/journal.pone.0126229; Nature Scientific Reports 2018 8:688; Neurology 2008 doi.org/10.1212/01.wnl.0000319696.14225.67; Curr Drug Targets 2014 15(4):423-31.
Target
Human HSPB1 / HSP27
Synonyms
HSPB1 | 28 kDa heat shock protein | Heat shock 27 kDa protein | Heat shock 27kD protein 1 | Heat shock 27kDa protein 1 | HSP27 | HSP28 | HSP 27 | Hsp25 | SRP27 | Stress-responsive protein 27 | CMT2F | Heat shock 27 kd protein | Heat shock protein beta-1 | HMN2B | HS.76067
Host
Mouse
Reactivity
Human
(tested or 100% immunogen sequence identity)
Clonality
Monoclonal
Conjugations
Unconjugated
Modifications
Unmodified
Applications
- IHC - Paraffin (10 µg/ml)
- IHC - Frozen
- Western blot
- Immunoprecipitation
- ELISA
|
Performing IHC? See our complete line of Immunohistochemistry Reagents including antigen retrieval solutions, blocking agents
ABC Detection Kits and polymers, biotinylated secondary antibodies, substrates and more.
|
Presentation
PBS, 0.08% Sodium Azide
Restrictions
For research use only. Intended for use by laboratory professionals.
About HSPB1 / HSP27
Validation
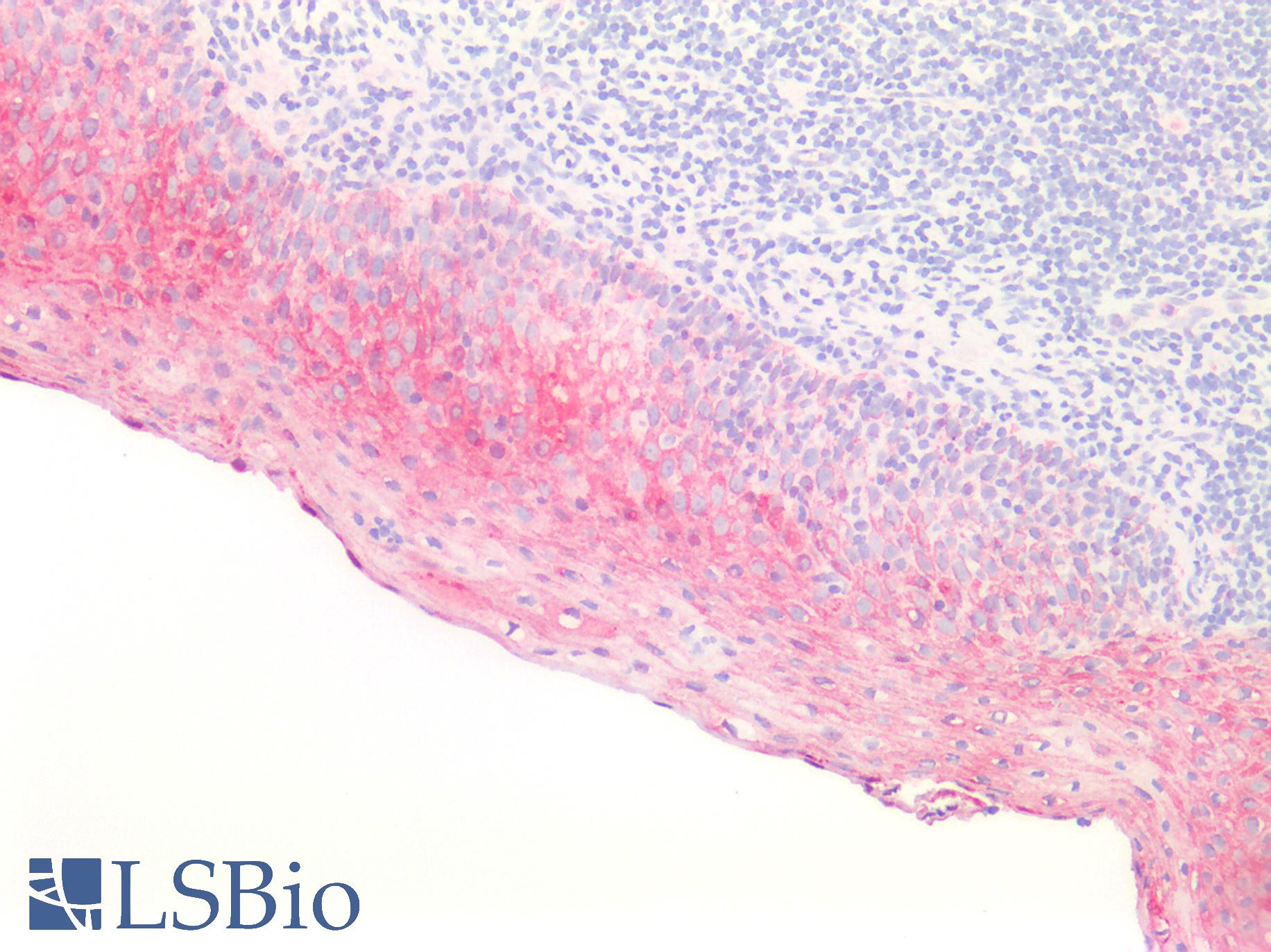
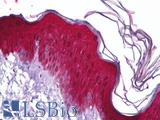
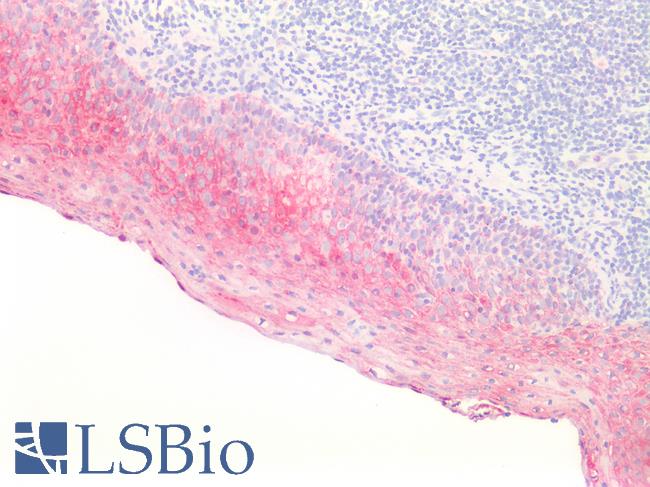
Human Tonsil, Squamous: Formalin-Fixed, Paraffin-Embedded (FFPE)
Human Tonsil, Squamous: Formalin-Fixed, Paraffin-Embedded (FFPE)
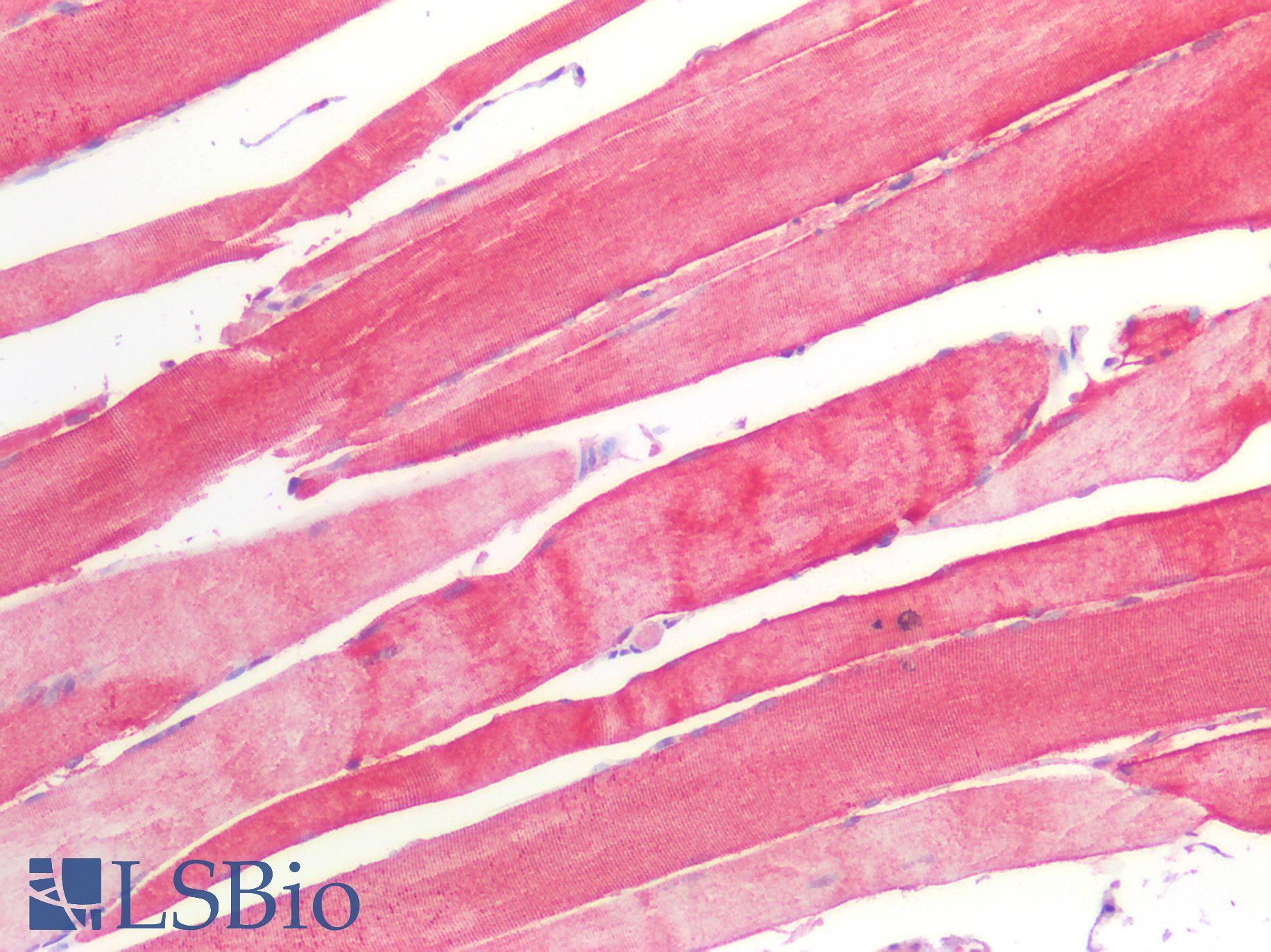
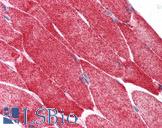
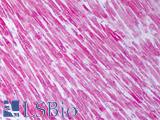
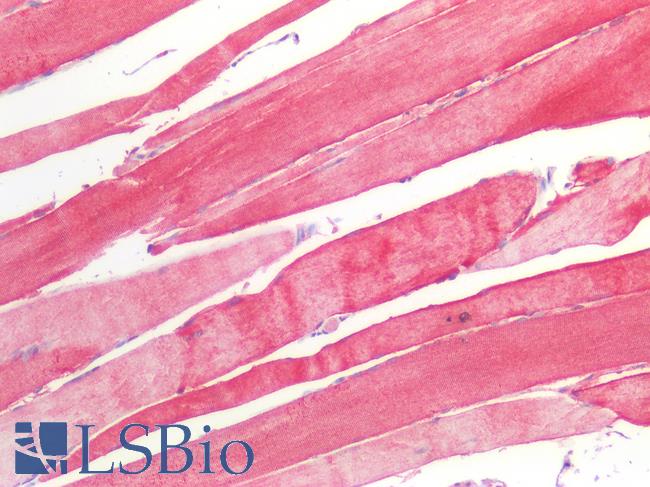
Human Skeletal Muscle: Formalin-Fixed, Paraffin-Embedded (FFPE)
Human Skeletal Muscle: Formalin-Fixed, Paraffin-Embedded (FFPE)
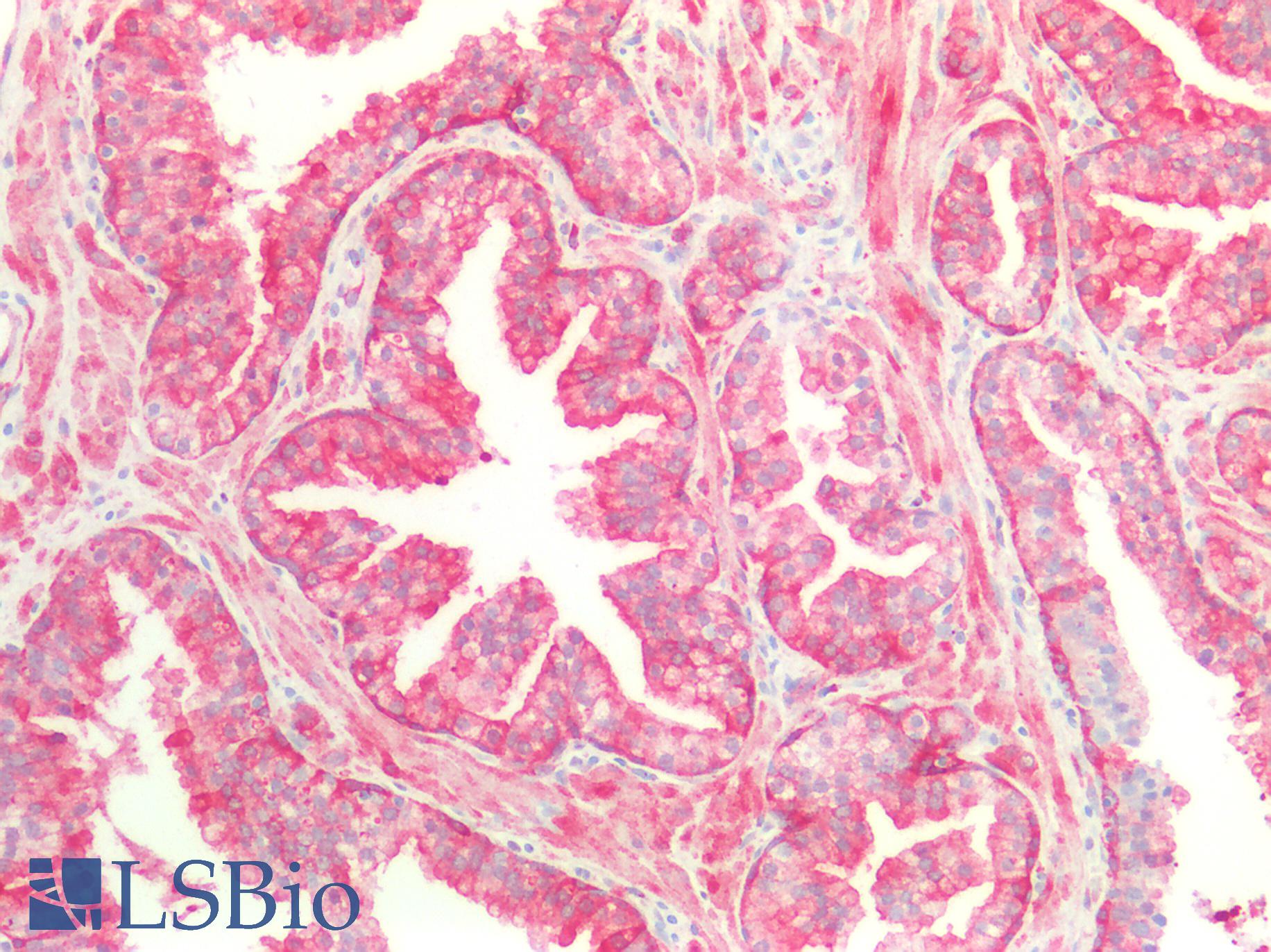
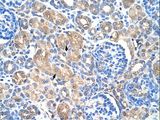
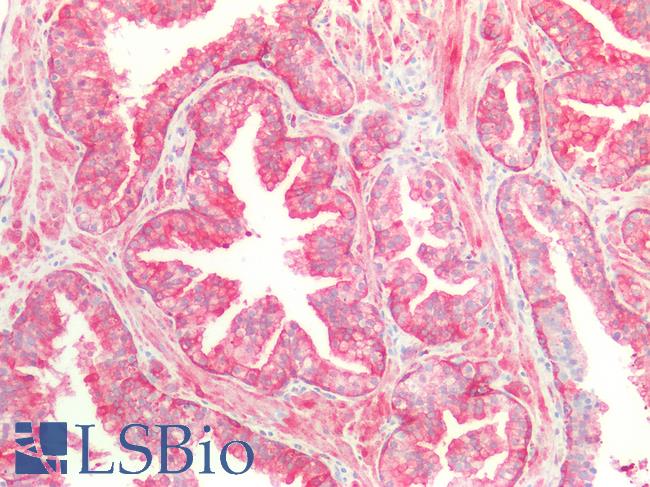
Human Prostate: Formalin-Fixed, Paraffin-Embedded (FFPE)
Human Prostate: Formalin-Fixed, Paraffin-Embedded (FFPE)
See More About...
LSBio Ratings
PathPlus™ HSPB1 / HSP27 Antibody for IHC, WB/Western, IP, ELISA LS-B16430 has an LSBio Rating of
Laboratory Validation Score (5)
Learn more about The LSBio Ratings Algorithm
Publications (0)
Customer Reviews (0)
Featured Products
Species:
Human
Applications:
IHC, IHC - Paraffin, Western blot, ELISA
Species:
Human, Mouse, Rat
Applications:
IHC, IHC - Paraffin, Western blot, Peptide Enzyme-Linked Immunosorbent Assay
Species:
Human
Applications:
IHC, IHC - Paraffin, Western blot, Immunoprecipitation, ELISA
Species:
Human
Applications:
IHC, IHC - Paraffin, Western blot
Species:
Turkey, Human
Applications:
IHC, IHC - Paraffin, IHC - Frozen, ICC, Western blot
Species:
Human, Monkey, Bovine, Guinea pig, Pig
Applications:
Western blot
Request SDS/MSDS
To request an SDS/MSDS form for this product, please contact our Technical Support department at:
Technical.Support@LSBio.com
Requested From: United States
Date Requested: 11/24/2024
Date Requested: 11/24/2024